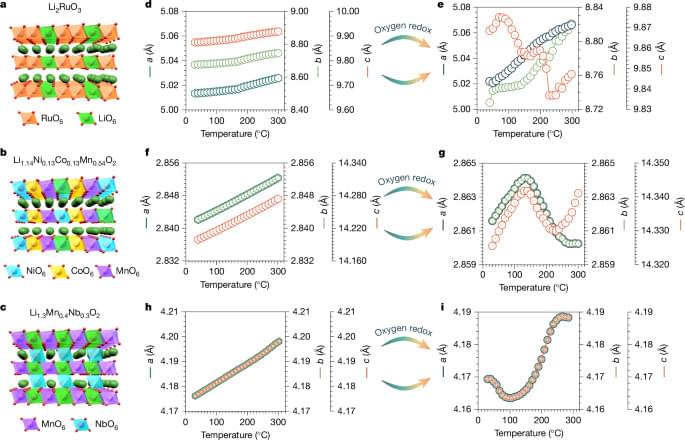
Using operando electrochemical processes, we found a way to restore oxygen-redox active materials exhibiting structural and voltage decay to their pristine state, providing a framework for the design of functional materials with zero thermal expansion.
For the first time, surgeons have successfully performed a remarkable new heart transplant in which the donor organ never skips a beat in the process, reducing the damage that can occur during such a complex operation. It ushers in a new era of more successful heart transplant surgery.
A team of surgeons at the National Taiwan University Hospital (NTUH) in Taipei undertook the revolutionary operation, during which the donor heart continues beating between the organ removal and transplantation stages. Traditionally, the donor heart would be removed and preserved in cold storage to reduce its workload – during this stage, it’s considered “ischemic time,” or the period during which the organ is cut off from blood supply. This comes with the risk of heart damage and rejection once it’s transplanted into a recipient.
When the heart is deprived of blood, ischemia – a shortage of oxygen – can damage its muscle tissue, or myocardium, reducing function and health once it is transplanted. While an organ set for transplant rarely endures more than a few hours in ischemic time, it can still lead to myocardial damage.
The rise of large language models like ChatGPT that can churn out computer code has led to a new term — vibe coding — for people who create software by asking AI to do it for them
In a physics first, a team including scientists from the National Institute of Standards and Technology (NIST) has created a way to make beams of neutrons travel in curves. These Airy beams (named for English scientist George Airy), which the team created using a custom-built device, could enhance neutrons’ ability to reveal useful information about materials ranging from pharmaceuticals to perfumes to pesticides — in part because the beams can bend around obstacles.
“We’ve known about these strange, self-steering wave patterns for a while, but until now, no one had ever made them with neutrons,” said NIST’s Michael Huber, one of the paper’s authors. “This opens up a whole new way to control neutron beams, which could help us see inside materials or explore some big questions in physics.”
A paper announcing the findings appears today in Physical Review Letters. The team was led by the University at Buffalo’s Dusan Sarenac, and coauthors from the Institute for Quantum Computing (IQC) at the University of Waterloo in Canada built the custom device that helped create the Airy beam. The team also includes scientists from the University of Maryland, Oak Ridge National Laboratory, Switzerland’s Paul Scherrer Institut, and Germany’s Jülich Center for Neutron Science at Heinz Maier-Leibnitz Zentrum.
We characterized the 3’ end sequence of the lncMN3 transcript using rapid amplification of cDNA ends (RACE) and we confirmed the existence of the annotated isoform (Appendix Fig. S1A).
The analysis of the expression profile of lncMN3 during mESCs in vitro differentiation to MNs indicated that while it is not expressed in mESCs, it starts to be present in embryoid bodies at day 5 (EB5), reaches its maximum in EBs at day 6 (EB6) and decreases in the mixed population containing MNs (DIV3) obtained after cells dissociation (Fig. 1B). The observed decrease in expression is probably caused by a dilution effect due to the experimental protocol used for MN differentiation (Wichterle and Peljto, 2008) rather than to a real down-regulation. In fact, the MN population obtained upon EBs dissociation accounts for 40% of the mixed neural cell population; moreover, in contrast to the mixed population which continues to divide, MNs are postmitotic cells and their amount is diluted as differentiation proceeds (Capauto et al, 2018).
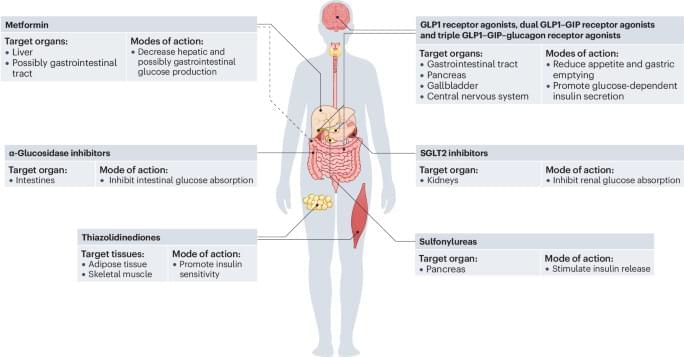
This Review covers the mechanistic relationship between hyperinsulinaemia and insulin resistance in type 2 diabetes mellitus. The article discusses how changes in insulin signalling affect metabolism and disease progression in various organs and cell types.
Superfluous axons are removed from muscle fibers through temporal changes in mRNA translation.
Real-world social cognition requires processing and adapting to multiple dynamic information streams. Interpreting neural activity in such ecological conditions remains a key challenge for neuroscience. This study leverages advancements in de-noising techniques and multivariate modeling to extract interpretable EEG signals from pairs of participants (male-male, female-female, and male-female) engaged in spontaneous dyadic dance. Using multivariate temporal response functions (mTRFs), we investigated how music acoustics, self-generated kinematics, other-generated kinematics, and social coordination uniquely contributed to EEG activity. Electromyogram recordings from ocular, face, and neck muscles were also modeled to control for artifacts. The mTRFs effectively disentangled neural signals associated with four processes: (I) auditory tracking of music, (II) control of self-generated movements, (III) visual monitoring of partner movements, and (IV) visual tracking of social coordination. We show that the first three neural signals are driven by event-related potentials: the P50-N100-P200 triggered by acoustic events, the central lateralized movement-related cortical potentials triggered by movement initiation, and the occipital N170 triggered by movement observation. Notably, the (previously unknown) neural marker of social coordination encodes the spatiotemporal alignment between dancers, surpassing the encoding of self-or partner-related kinematics taken alone. This marker emerges when partners can see each other, exhibits a topographical distribution over occipital areas, and is specifically driven by movement observation rather than initiation. Using data-driven kinematic decomposition, we further show that vertical bounce movements best drive observers’ EEG activity. These findings highlight the potential of real-world neuroimaging, combined with multivariate modeling, to uncover the mechanisms underlying complex yet natural social behaviors.
Significance statement Real-world brain function involves integrating multiple information streams simultaneously. However, due to a shortfall of computational methods, laboratory-based neuroscience often examines neural processes in isolation. Using multivariate modeling of EEG data from pairs of participants freely dancing to music, we demonstrate that it is possible to tease apart physiologically established neural processes associated with music perception, motor control, and observation of a partner’s movement. Crucially, we identify a previously unknown neural marker of social coordination that encodes the spatiotemporal alignment between dancers, beyond self-or partner-related kinematics alone. These findings highlight the potential of computational neuroscience to uncover the biological mechanisms underlying real-world social and motor behaviors, advancing our understanding of how the brain supports dynamic and interactive activities.
Controlling the uniformity in size and quantity of macroscopic three-dimensional (3D) DNA crystals is essential for their integration into complex systems and broader applications. However, achieving such control remains a major challenge in DNA nanotechnology. Here, we present a novel strategy for synthesizing monodisperse 3D DNA single crystals using microfluidic double-emulsion droplets as nanoliter-scale microreactors. These uniformly sized droplets can shrink and swell without leaking their inner contents, allowing the concentration of the DNA solution inside to be adjusted. The confined volume ensures that, once a crystal seed forms, it rapidly consumes the available DNA material, preventing the formation of additional crystals within the same droplet. This approach enables precise control over crystal growth, resulting in a yield of one DNA single crystal per droplet, with a success rate of up to 98.6% ± 0.9%. The resulting DNA crystals exhibit controlled sizes, ranging from 19.3 ± 0.9 μm to 56.8 ± 2.6 μm. Moreover, this method can be applied to the controlled growth of various types of DNA crystals. Our study provides a new pathway for DNA crystal self-assembly and microengineering.