Insights from the 1st World Longevity Summit show how social ties, daily movement, plant-forward meals, and purpose improve healthy life expectancy.


A research team led by Professor Eijiro Miyako at the Graduate School of Advanced Science and Technology, Japan Advanced Institute of Science and Technology (JAIST), has discovered that the marine bacterium Photobacterium angustum demonstrates remarkable therapeutic efficacy against colorectal cancer.
Through screening of multiple marine bacterial strains, the researchers found that P. angustum, in its natural, non-engineered form, selectively accumulates in tumor tissues and induces both direct tumor lysis and robust immune activation.
In mouse models, intravenously administered P. angustum showed high tumor tropism while exhibiting minimal colonization of vital organs except the liver, with no hematological abnormalities or histological toxicity observed.


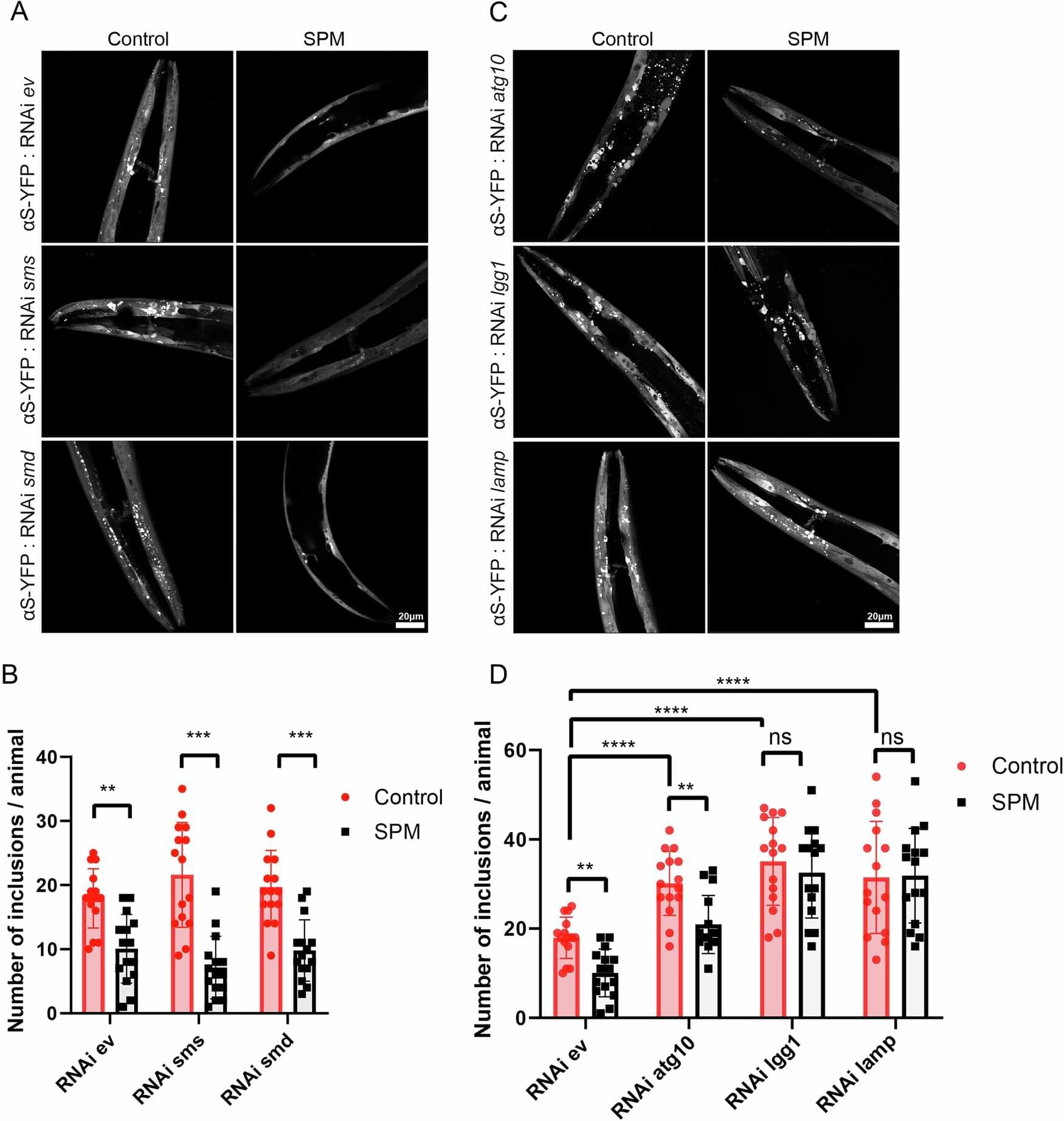
Researchers at the Paul Scherrer Institute PSI have clarified how spermine—a small molecule that regulates many processes in the body’s cells—can guard against diseases such as Alzheimer’s and Parkinson’s: It renders certain proteins harmless by acting a bit like cheese on noodles, making them clump together. This discovery could help combat such diseases. The study has now been published in the journal Nature Communications.
Our life expectancy keeps rising—and as it does, age-related illnesses, including neurodegenerative diseases such as Alzheimer’s and Parkinson’s, are becoming increasingly common. These diseases are caused by accumulations in the brain of harmful protein structures consisting of incorrectly folded amyloid proteins. Their shape is reminiscent of fibers or spaghetti. To date, there is no effective therapy to prevent or eliminate such accumulations.
Yet a naturally occurring molecule in the body called spermine offers hope. In experiments, researchers led by study leader Jinghui Luo, in the Center for Life Sciences at the Paul Scherrer Institute PSI, have discovered that this substance is capable of extending the lifespan of small nematode worms, improving their mobility in old age, and strengthening the powerhouses of their cells—the mitochondria. Specifically, the researchers observed how spermine helps the body’s immune system eliminate nerve-damaging accumulations of amyloid proteins.



For years, Rick Stevens, a computer scientist at Argonne National Laboratory, pushed the notion of transforming scientific computing with artificial intelligence.
But even as Mr. Stevens worked toward that goal, government labs like Argonne — created in 1946 and sponsored by the Department of Energy — often took five years or more to develop powerful supercomputers that can be used for A.I. research. Mr. Stevens watched as companies like Amazon, Microsoft and Elon Musk’s xAI made faster gains by installing large A.I. systems in a matter of months.