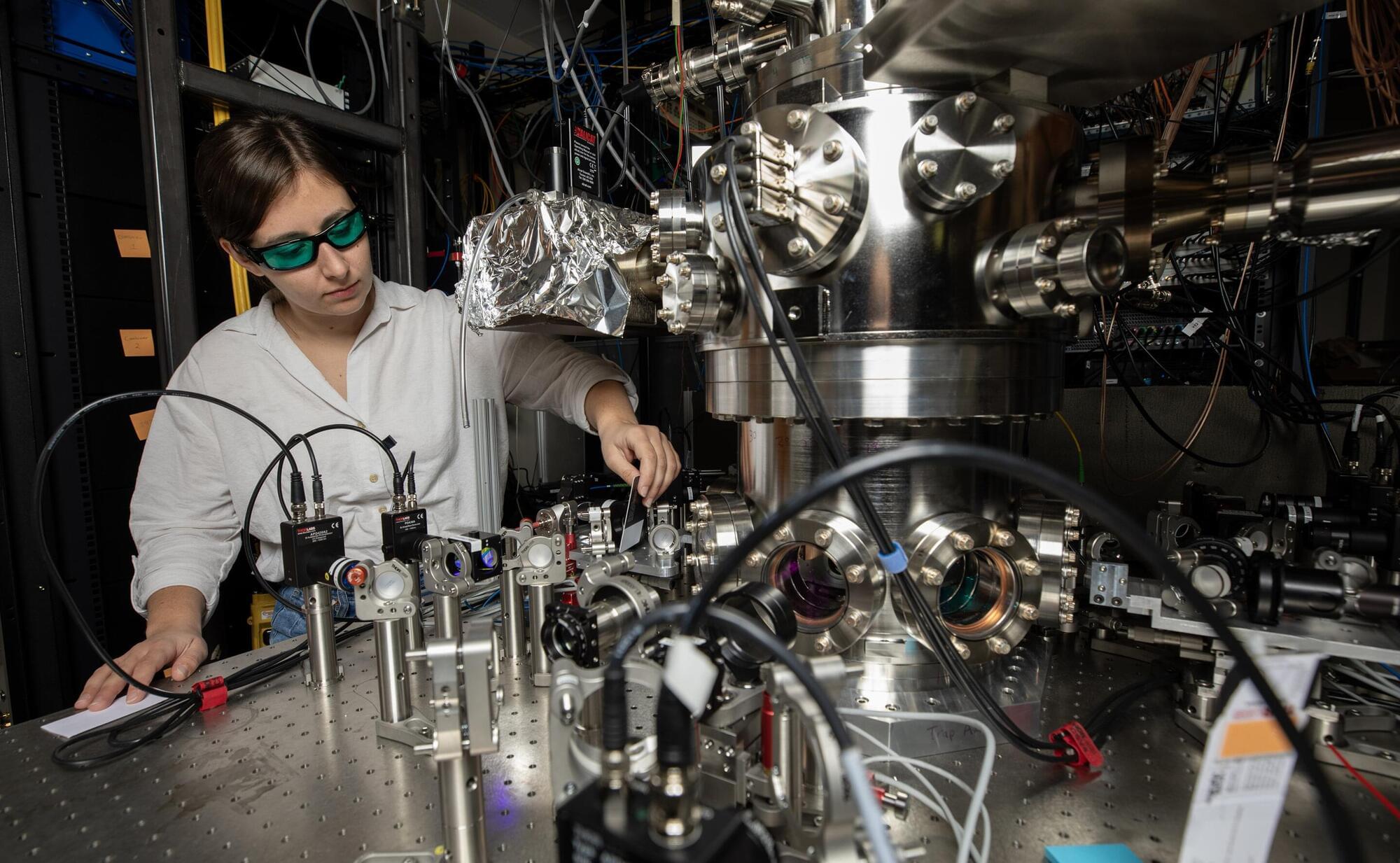
Research in the lab of UC Santa Barbara materials professor Stephen Wilson is focused on understanding the fundamental physics behind unusual states of matter and developing materials that can host the kinds of properties needed for quantum functionalities.
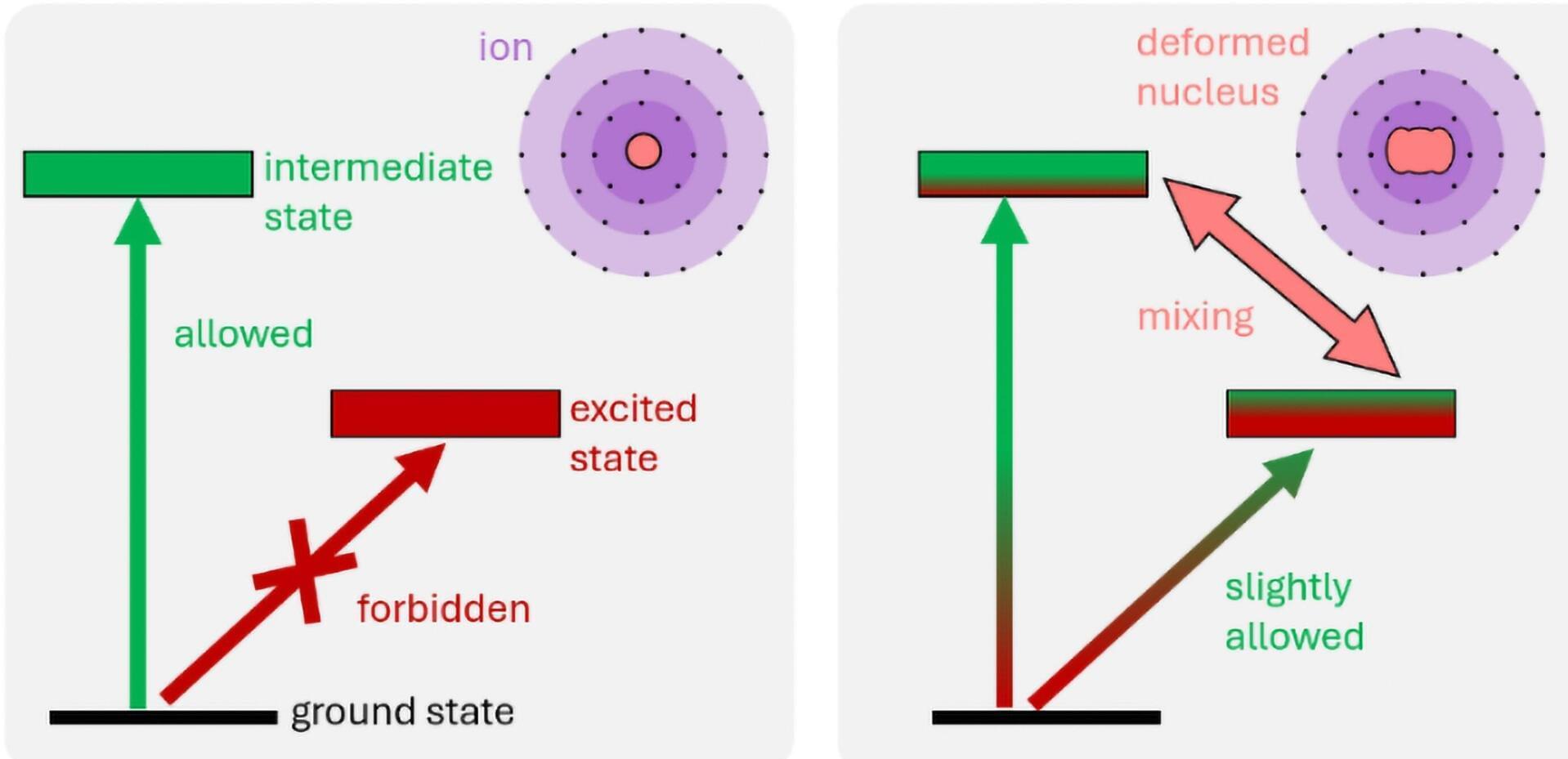
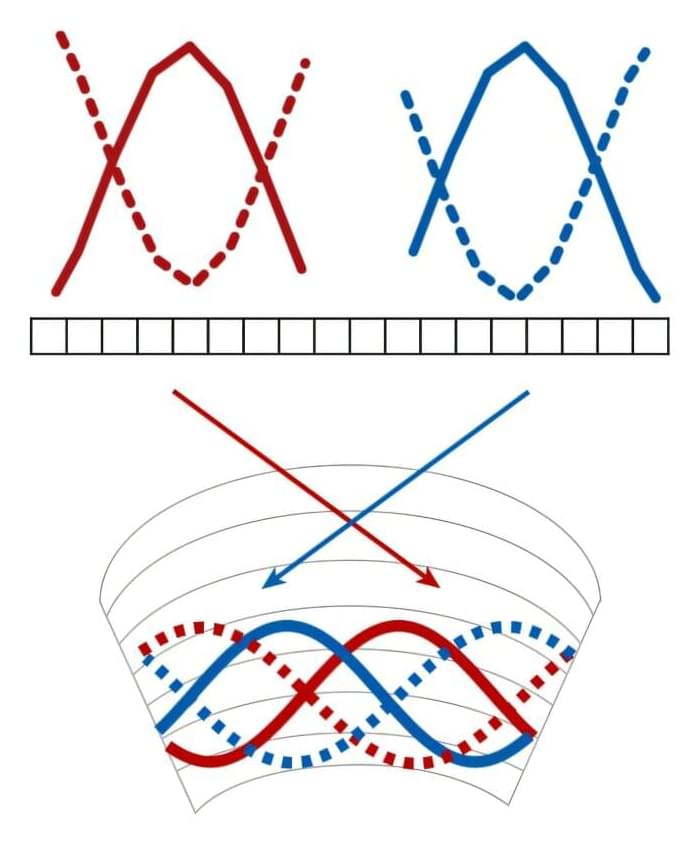
In a paper published in Nature Materials, Wilson’s lab group has reported on an innovative way to use a phenomenon referred to as frustration of long-range order in a material system to engineer unconventional magnetic states with potential relevance for quantum technologies.
At the same time, Wilson emphasized, “This is fundamental science aimed at addressing a basic question. It’s meant to probe what physics may be possible for future devices.”