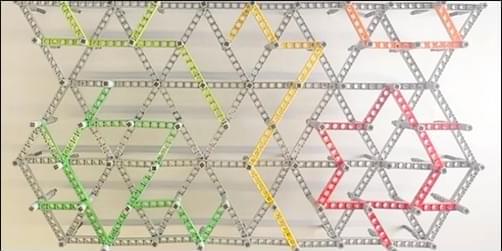
A research team has developed a triangular mechanical network that can squeeze and wiggle in a multitude of preprogrammed ways [1]. The metamaterial design—realized in experiments with various materials, including Legos—may have applications from shock absorption to protein modeling. But the researchers also demonstrated that their structures can solve problems in matrix algebra. Performing computations in materials without converting information to electrical signals could be useful when durability and energy efficiency are more important than computing power, for example, in components of some soft robots.
Recent work showed that a mechanical system can perform similar computations [2]. However, this previous demonstration was limited in the number of inputs and outputs that it could accommodate, says Yair Shokef of Tel Aviv University in Israel. It also had rather large components that made it difficult to adapt to different applications.
Shokef and his colleagues, who produced the latest demonstration, built their 2D networks from equilateral triangles. Each triangle consisted of rigid beams with hinge points at each vertex and at the center of each side, for a total of three so-called corner nodes and three edge nodes per triangle. Importantly, each triangle had one or two “bonds”—beams that connected edge nodes and that determined the ways in which the triangle could be distorted or flexed.