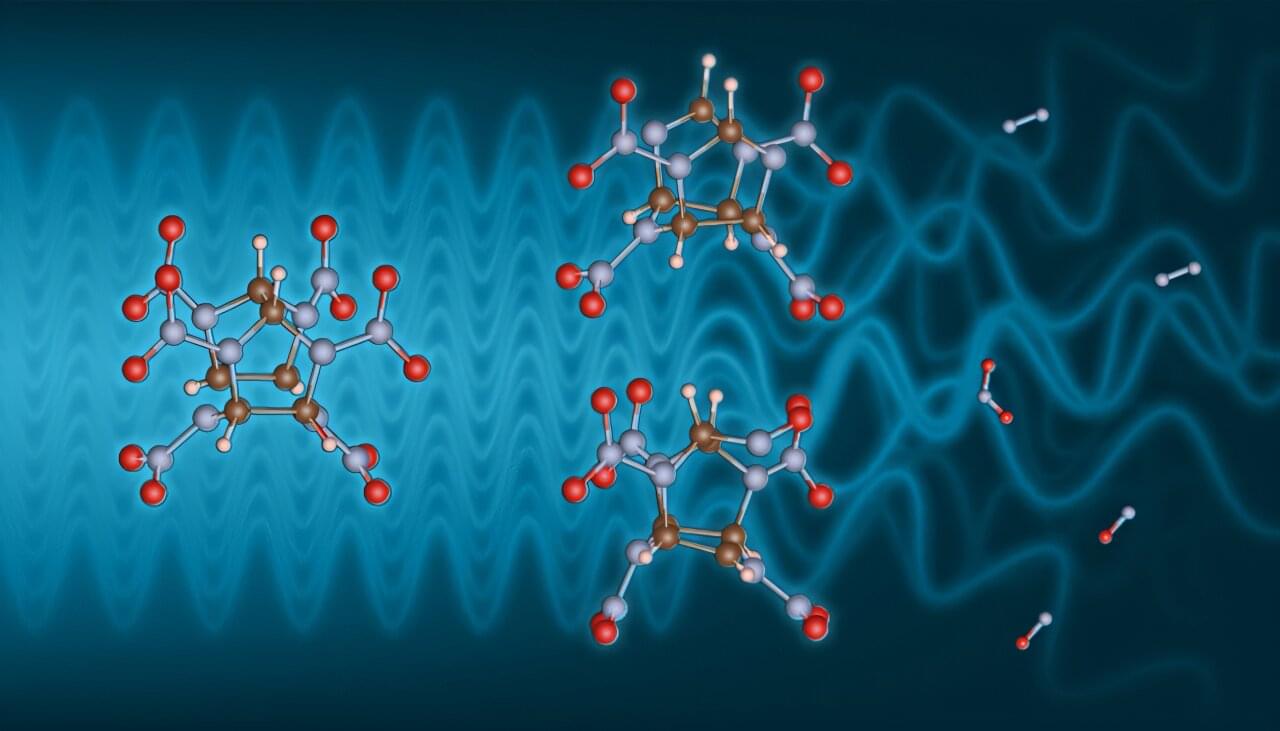
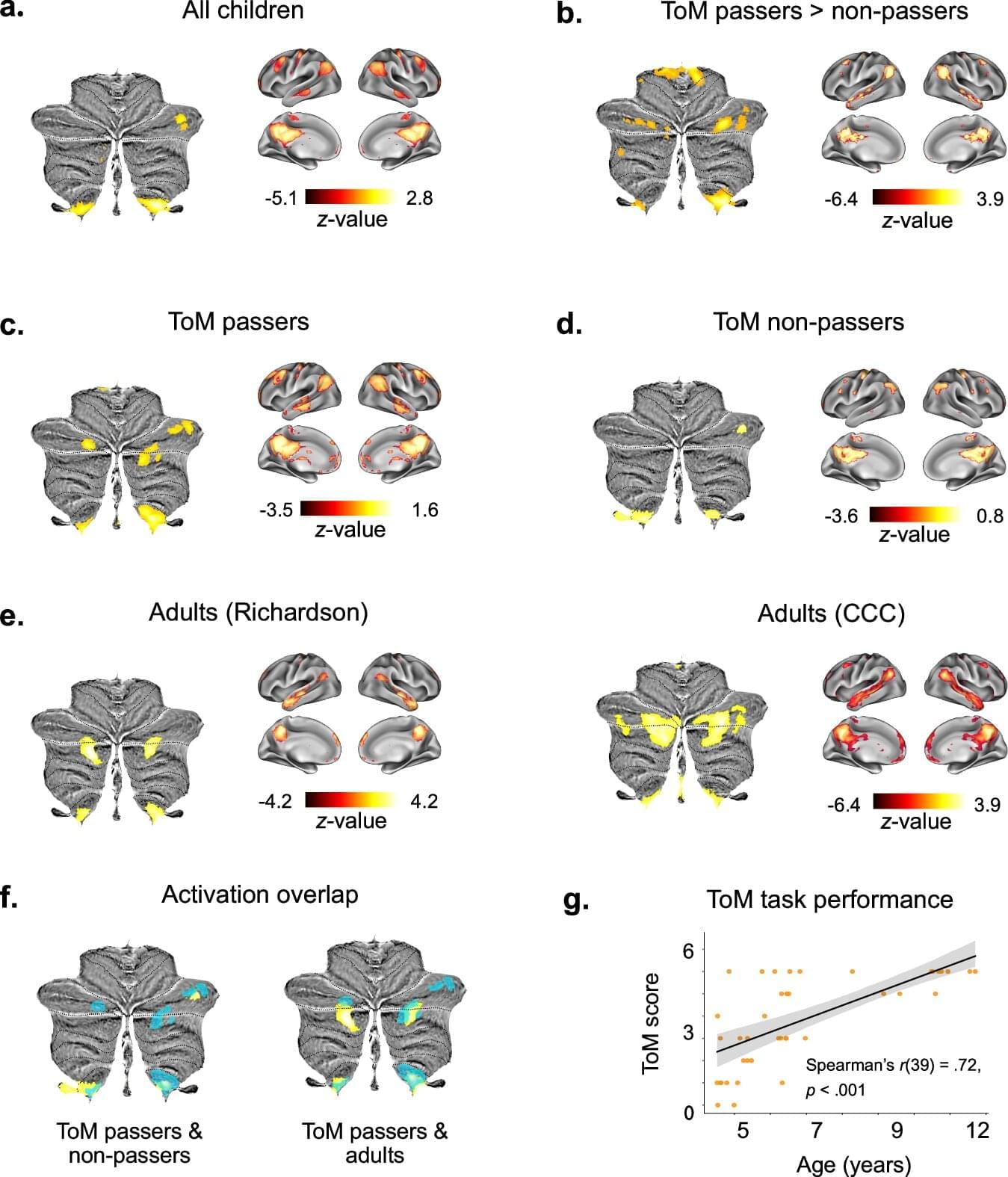
Safe and effective high explosives are critical to Lawrence Livermore National Laboratory’s (LLNL) mission of stockpile stewardship. It is relatively simple to study the composition of such material before a detonation or examine the soot-like remnants afterward. But the chemistry in between, which dictates much of the detonation process, evades experimental interrogation as it passes by in a few nanoseconds or less.
In a study published in the Proceedings of the National Academy of Sciences, researchers from SLAC National Accelerator Laboratory and LLNL triggered a slow decomposition of a high explosive and measured the effects on the molecules within it. The work provides the proof of concept for a process that could be extended to examine ultra-fast dynamic chemistry during detonations and illuminates intermediate structures that have never been experimentally seen before.
At the Stanford Synchrotron Radiation Lightsource, the team used X-rays to both trigger the chemical reactions involved in decomposition and measure the results.