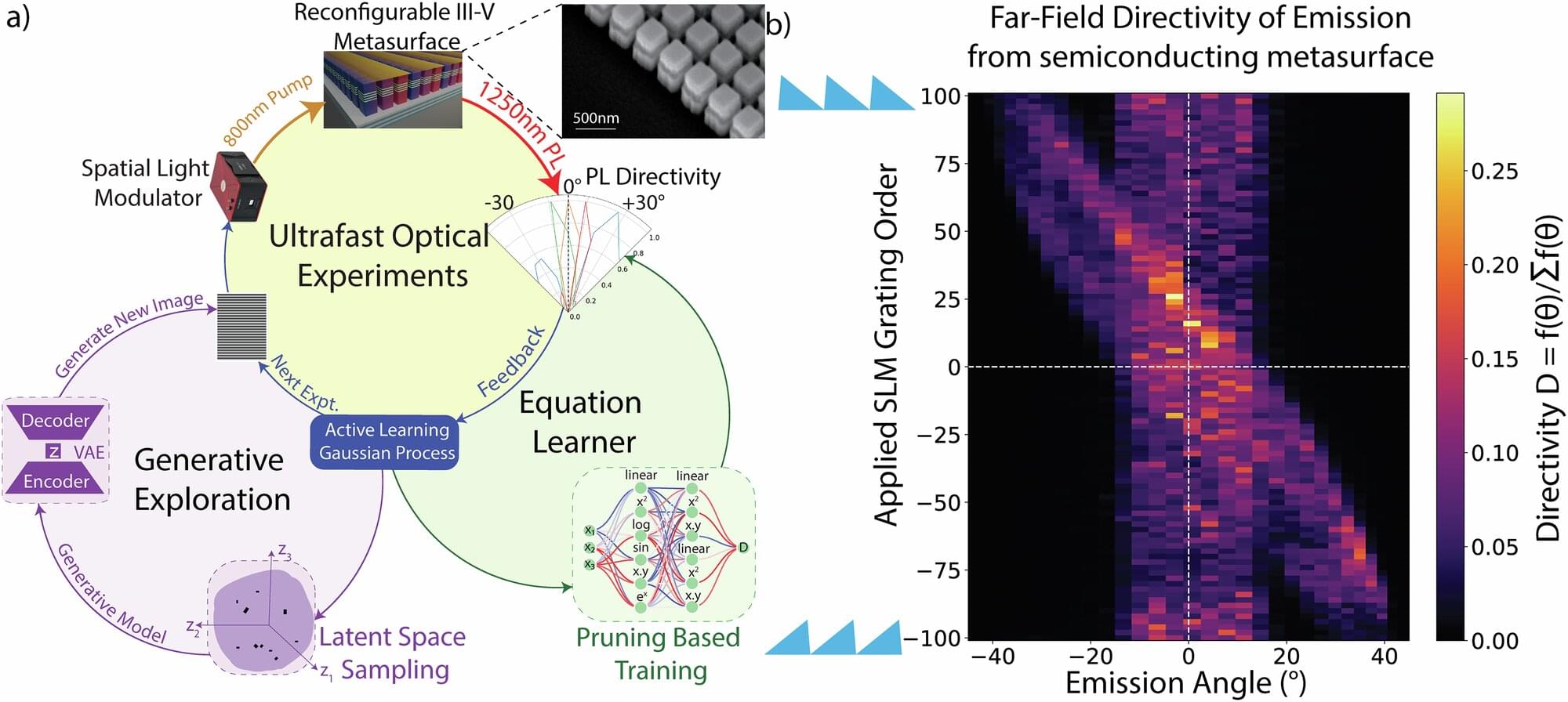
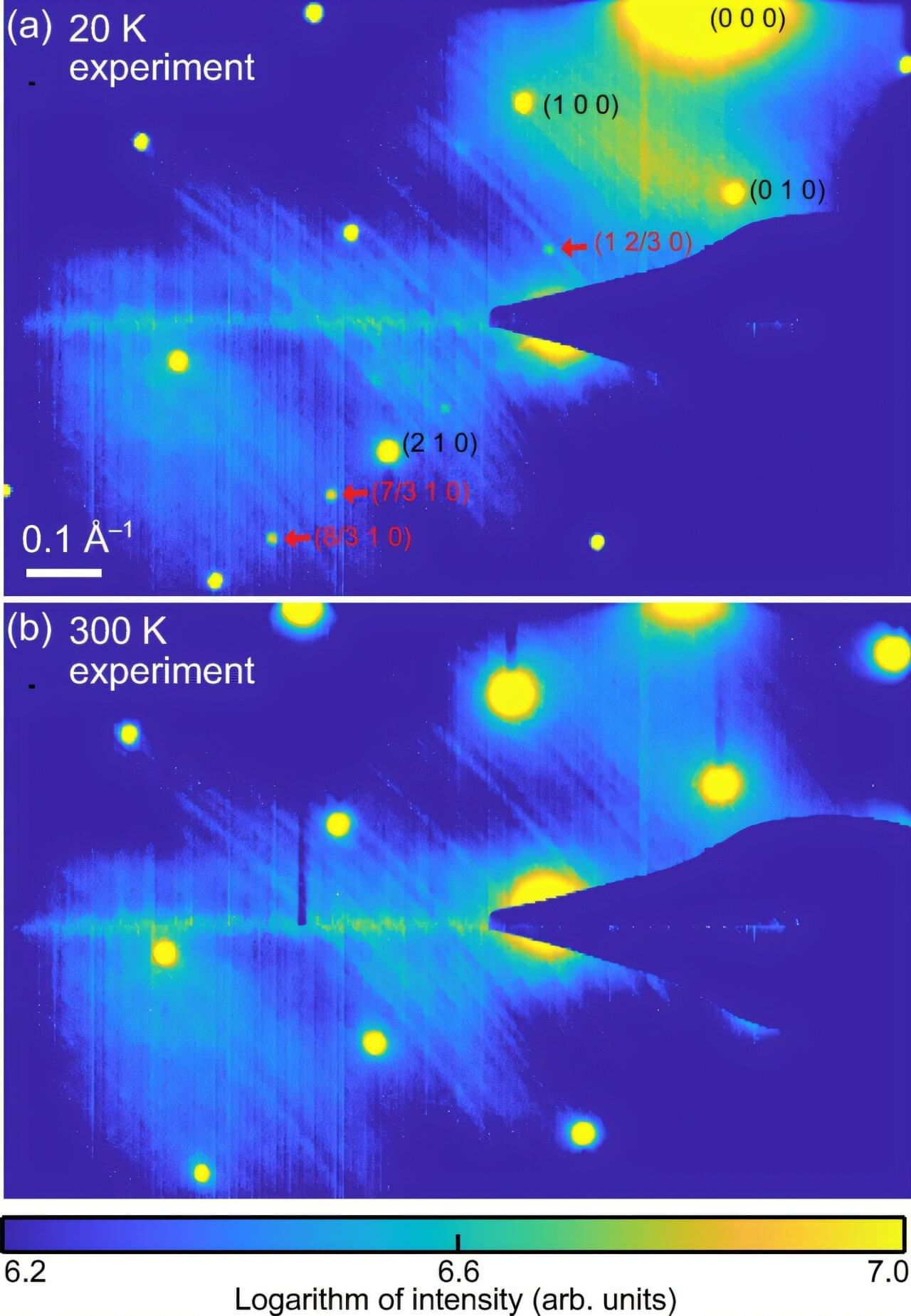
In 2023, a team of physicists from Sandia National Laboratories announced a major discovery: a way to steer LED light. If refined, it could mean someday replacing lasers with cheaper, smaller, more energy-efficient LEDs in countless technologies, from UPC scanners and holographic projectors to self-driving cars. The team assumed it would take years of meticulous experimentation to refine their technique.
Now the same researchers have reported that a trio of artificial intelligence labmates has improved their best results fourfold. It took about five hours.
The resulting paper, now published in Nature Communications, shows how AI is advancing beyond a mere automation tool toward becoming a powerful engine for clear, comprehensible scientific discovery.