An early lab-dish study in cancer cells suggests microplastics can persist through cell division and may contribute to cancer spread, when they’re in tumors.


Calling all stargazers: Oregon is now home to the largest Dark Sky Sanctuary in the world.
Earlier this month, DarkSky International certified a remote, 2.5 million-acre area in the southeastern part of the state. From this rugged swath of high desert landscape dotted with sagebrush, visitors who stay up late can see large numbers of stars, planets and other celestial bodies.
“It’s surprising sometimes to see that many stars all at once,” says Bob Hackett, executive director of Travel Southern Oregon, to the Guardian’s Dani Anguiano. “It catches you, and it makes you pause because you feel like you can touch it … That vastness of the whole cosmos up there—it almost makes you get closer to the people you’re with on the ground.”

PORTLAND, Maine (AP) — Avian influenza is killing tens of thousands of seals and sea lions in different corners of the world, disrupting ecosystems and flummoxing scientists who don’t see a clear way to slow the devastating virus.
The worldwide bird flu outbreak that began in 2020 has led to the deaths of millions of domesticated birds and spread to wildlife all over the globe. This virus isn’t thought to be a major threat to humans, but its spread in farming operations and wild ecosystems has caused widespread economic turmoil and environmental disruptions.
Seals and sea lions, in places as far apart as Maine and Chile, appear to be especially vulnerable to the disease, scientists said. The virus has been detected in seals on the east and west coasts of the U.S., leading to deaths of more than 300 seals in New England and a handful more in Puget Sound in Washington. The situation is even more dire in South America, where more than 20,000 sea lions have died in Chile and Peru and thousands of elephant seals have died in Argentina.
Electrons swarm in a soup of quantum entanglement in a new class of materials called strange metals.
From NVIDIA efficient video diffusion models via content-frame motion-latent decomposition.
From NVIDIA
Efficient video diffusion models via content-frame motion-latent decomposition.
Video diffusion models have recently made great progress in generation quality, but are still limited by the high memory and computational requirements.
Join the discussion on this paper page.
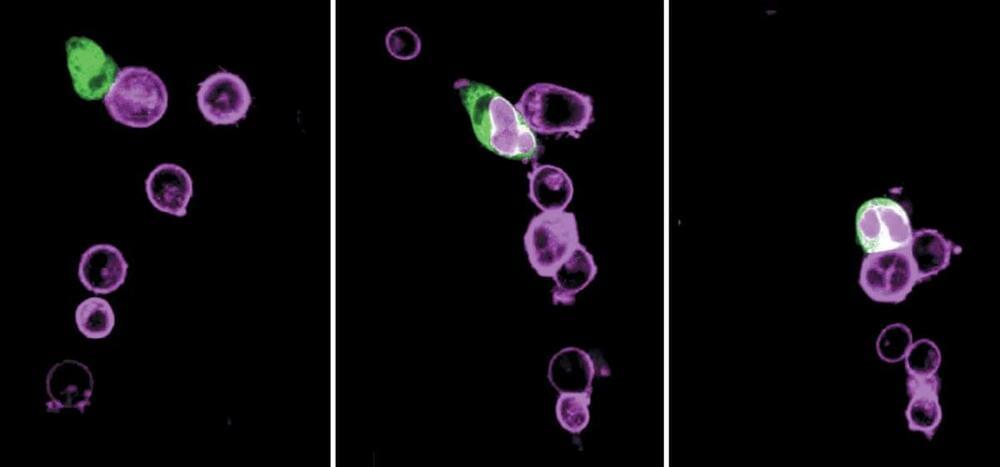
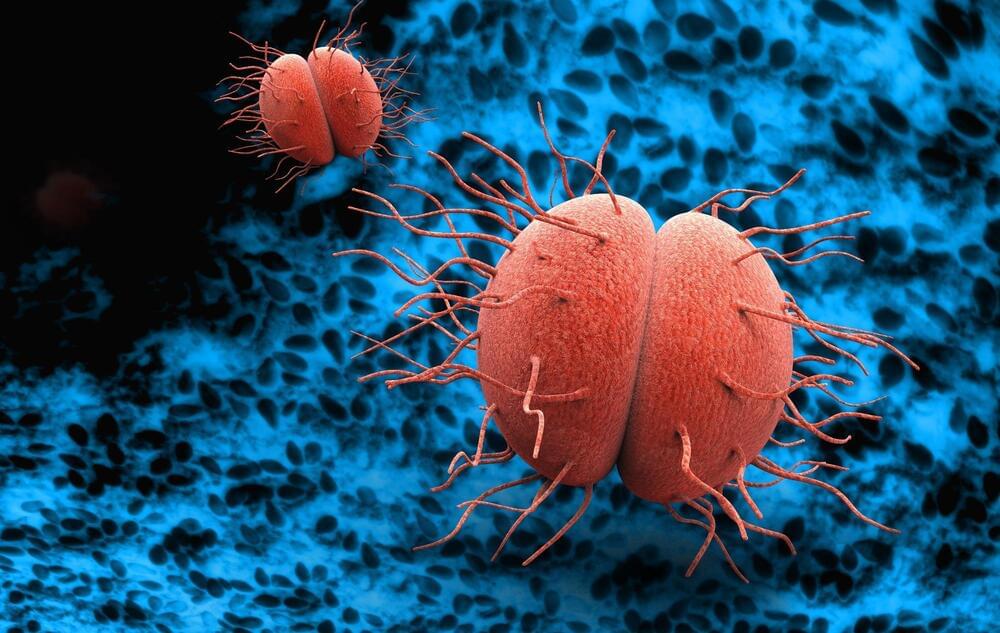
🦠💊🌍 https://www.news-medical.net/news/20240321/Rising-antimicrob…action.asp
Review delves into the rising challenge of antimicrobial resistance in sexually transmitted infections, underscoring the need for innovative treatments and the critical role of global surveillance in managing diseases like gonorrhea and syphilis.
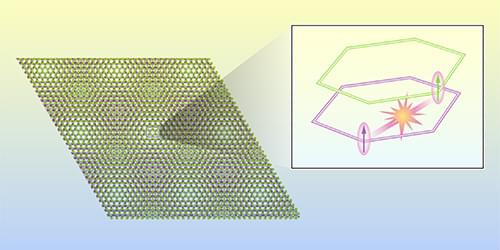
The unexpected observation of an aligned spin polarization in certain twisted semiconductor bilayers calls for improved models of these systems.
If you take two overlapping tiled patterns and rotate one with respect to the other, new patterns will emerge. This motif has been used in art and architecture for millennia. Over the past 15 years, materials physicists have used a similar strategy to realize new material properties. In one implementation, two material monolayers with a hexagonal atomic lattice are overlaid with an angle between the two lattices, resulting in an additional long-range lattice structure known as a moiré pattern. In 2021, scientists observed the so-called quantum anomalous Hall (QAH) effect in such a twisted bilayer, formed of MoTe2 and WSe2 monolayers [1]. Now Zui Tao at Cornell University and colleagues have used optical spectroscopy to study the interaction between these two monolayers when they are in the QAH state [2].
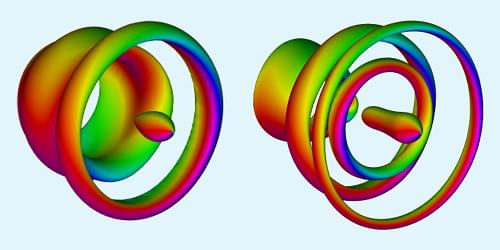
Loop-shaped structures called vortex rings are remarkably stable and are seen throughout nature, appearing as bubble rings blown by dolphins and smoke rings emitted by erupting volcanoes. Recently, scientists observed vortex rings made from electron spins in magnetic materials. These structures have properties that make them attractive for use in energy-efficient data storage and processing. Now Yizhou Liu and Naoto Nagaosa at the RIKEN Center for Emergent Matter Science in Japan have proposed a way to create such magnetic vortex rings on demand [1].
Liu and Nagaosa considered a nanometer-scale cylinder made of a “chiral” magnetic material, one whose magnetic structure differs from that of its mirror image. The magnetic vortex rings that form in such a system have more diverse topologies and greater stability than those that form in other systems. In numerical simulations, the researchers injected a pulse of electric current into their chiral magnetic cylinder through a circular trench etched into the cylinder’s top surface. They then studied how the current pulse affected the material’s spin configurations.
Liu and Nagaosa observed a chain of interconnected magnetic vortex rings form along the length of the cylinder. Varying the duration and amplitude of the injected current pulse, they were able to control the topology of the vortex rings and their connections. The researchers say that the next step is for experimentalists to replicate these findings in the lab. They also suggest that their technique could be adapted to produce magnetic vortex rings in other physical systems, such as liquid crystals and Bose-Einstein condensates.