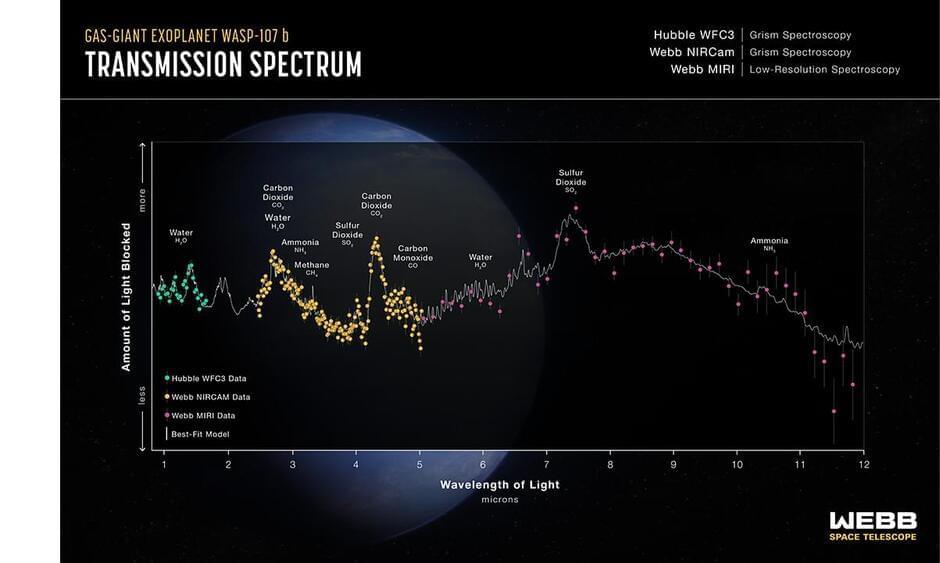
Why is the warm gas-giant exoplanet WASP-107 b so puffy? Two independent teams of researchers have an answer.
Data collected using NASA’s James Webb Space Telescope, combined with prior observations from NASA’s Hubble Space Telescope, show surprisingly little methane (CH4) in the planet’s atmosphere, indicating that the interior of WASP-107 b must be significantly hotter and the core much more massive than previously estimated.
The unexpectedly high temperature is thought to be a result of tidal heating caused by the planet’s slightly non-circular orbit, and can explain how WASP-107 b can be so inflated without resorting to extreme theories of how it formed.