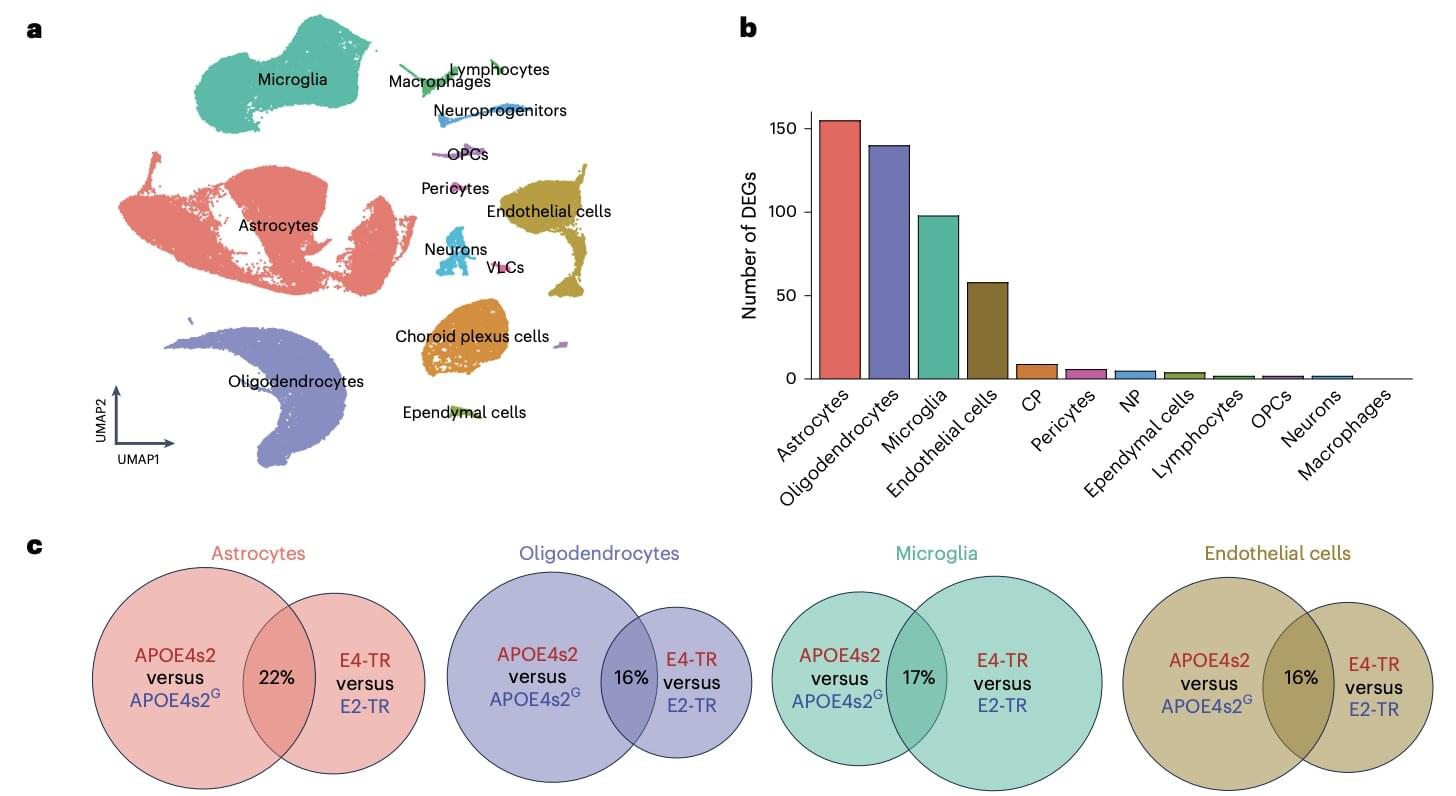
Nuclear power company TerraPower has passed the Nuclear Regulatory Commission staff’s final safety evaluation for a permit to build a reactor in Wyoming. The Washington-based company backed by Bill Gates and NVIDIA could be the first to deploy a utility-scale, next-generation reactor in America.
TerraPower’s Natrium design pairs a small modular reactor (SMR) with an integrated thermal battery. The SMR generates 345 megawatts of continuous electrical power. The thermal battery, which stores excess heat in molten salt, allows the system to surge its output to 500 megawatts for more than five hours, generating enough energy to power 400,000 homes at maximum capacity.
“Today is a momentous occasion for TerraPower, our project partners and the Natrium design,” said company CEO Chris Levesque in a statement issued Monday. The favorable assessment “reflects years of rigorous evaluation, thoughtful collaboration with the NRC, and an unwavering commitment to both safety and innovation.”