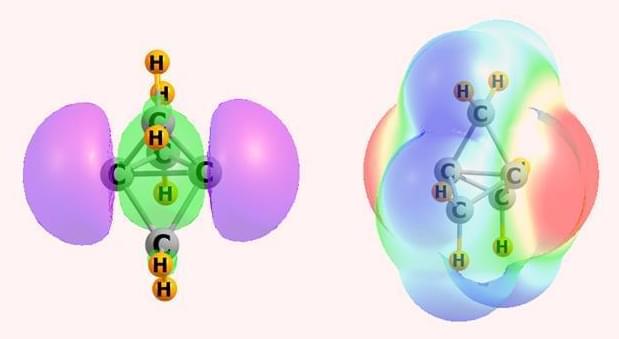
Scheiner and Zierkiewicz, however, have been studying apical carbon atoms in propellane and pyramidane molecules, where the bonding situation is rather different. Along with Mariusz Michalczyk, also at Wrocław University of Science and Technology, they’ve identified an electron-donating orbital – or pseudo lone pair – on these tetrahedral carbons.
While it clearly has a negative charge, Scheiner acknowledges that the nature of this electron-donating orbital could be up for debate. Nonetheless, it appears that this region of negative electrostatic potential can attract the σ-hole of an electrophile to form various non-covalent interactions including hydrogen, halogen, chalcogen, pnictogen and tetrel bonds.