This study offers models to better understand and describe progression of muscle fat fraction of the thighs and lower legs of patients with dysferlinopathy (LGMDR2) using clinical and radiologic data.
Background and Objectives.

We speak with neurologist Helen Bronte-Stewart, who conducted research that led to the development of a technology recently approved by the U.S. Food and Drug Administration.


Apple CEO Tim Cook told employees at an all-hands meeting that the AI revolution is “as big or bigger” than the internet, smartphones, cloud computing, and apps. According to Bloomberg’s Power On newsletter, Cook said, “Apple must do this,” adding that this is “ours to grab.” He expressed hopes that, though Apple has been relatively late in rolling out AI tools—Apple Intelligence was only unveiled in 2024 —it could still dominate its rivals.
“We’ve rarely been first,” the CEO told staff. “There was a PC before the Mac; there was a smartphone before the iPhone; there were many tablets before the iPad; there was an MP3 player before iPod.”
But Cook argued that Apple invented the “modern” versions of those products, adding: “This is how I feel about AI.” He also discussed practical steps Apple is taking to make these plans a reality. Cook said Apple is investing in AI in a “big way,” and that 40% of the 12,000 employees hired last year are set to work on research and development.

Jake Adler, the 21-year-old founder of the biotech and defense startup Pilgrim, literally put his own sweat and blood into developing the business, cutting his thighs to demonstrate the new technology. Surprisingly, this bloody effort paid off and he received $4.3 million from investors.
Pilgrim creates biotechnology for use on the battlefield, with plans to sell to the military and eventually civilians. Their flagship product is the Kingsfoil hemostatic bandage, which startup CEO Jake Adler cut both of his legs on camera to demonstrate.
We won’t publish the video and will avoid giving details. In short: Adler anesthetized his legs with lidocaine and used a biopsy tool to make two precise cuts. One of them was covered with Kingsfoil to stop the bleeding, and the other was used for a control comparison.

The evolutionary success of our species may have hinged on minute changes to our brain biochemistry after we diverged from the lineage leading to Neanderthals and Denisovans about half a million years ago.
Two of these tiny changes that set modern humans apart from Neanderthals and Denisovans affect the stability and genetic expression of the enzyme adenylosuccinate lyase, or ADSL. This enzyme is involved in the biosynthesis of purine, one of the fundamental building blocks of DNA, RNA, and other important biomolecules.
In a study published in PNAS, researchers from the Okinawa Institute of Science and Technology (OIST), Japan and the Max Planck Institute for Evolutionary Anthropology, Germany have discovered that these changes may play an important role in our behavior, contributing new pieces to the great puzzle of who we humans are and where we come from.
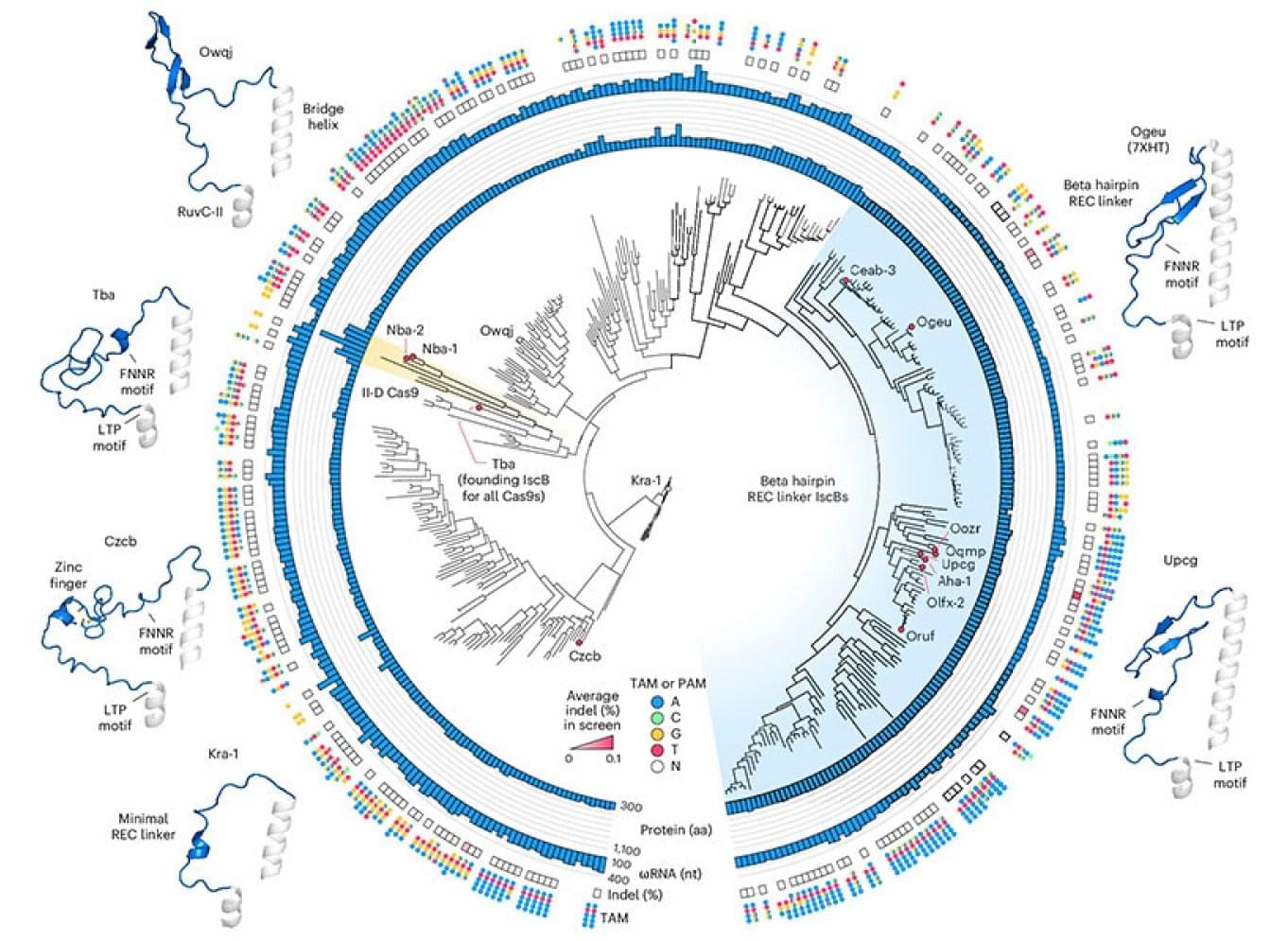
Scientists at the McGovern Institute for Brain Research at MIT and the Broad Institute of MIT and Harvard have re-engineered a compact RNA-guided enzyme they found in bacteria into an efficient, programmable editor of human DNA.
The protein they created, called NovaIscB, can be adapted to make precise changes to the genetic code, modulate the activity of specific genes, or carry out other editing tasks. Because its small size simplifies delivery to cells, NovaIscB’s developers say it is a promising candidate for developing gene therapies to treat or prevent disease.
The study was led by Feng Zhang, the James and Patricia Poitras Professor of Neuroscience at MIT who is also an investigator at the McGovern Institute and the Howard Hughes Medical Institute, and a core member of the Broad Institute. Zhang and his team reported their open-access work this month in the journal Nature Biotechnology.
Researchers at MIT and the Broad Institute, led by Professor Feng Zhang, redesign a compact RNA-guided enzyme from bacteria, making it an efficient editor of human DNA.