Colonized artificial reef structures could absorb the power of storms.



Using the James Webb Space Telescope, astronomers have for the first time observed a galaxy in the early universe growing from the inside out, a mere 700 million years after the Big Bang.
This galaxy, significantly smaller yet more mature than expected, demonstrates unique growth patterns with its dense core and rapidly forming star outskirts.
Astronomers have employed the NASA /ESA James Webb Space Telescope (JWST) to observe the ‘inside-out’ growth of a galaxy in the early universe, a mere 700 million years after the Big Bang.
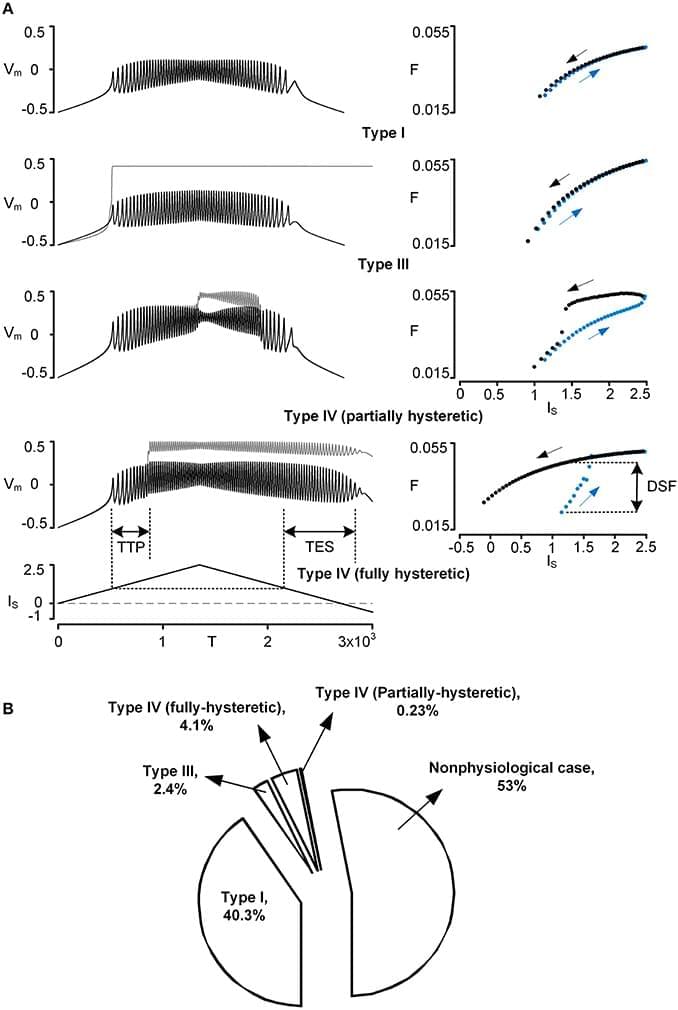
However, the effect of the PIC on firing outputs also depends on its location in the dendritic tree. To investigate the interaction between PIC neuromodulation and PIC location dependence, we used a two-compartment model that was biologically realistic in that it retains directional and frequency-dependent electrical coupling between the soma and the dendrites, as seen in multi-compartment models based on full anatomical reconstructions of motoneurons. Our two-compartment approach allowed us to systematically vary the coupling parameters between the soma and the dendrite to accurately reproduce the effect of location of the dendritic PIC on the generation of nonlinear (hysteretic) motoneuron firing patterns. Our results show that as a single parameter value for PIC activation was either increased or decreased by 20% from its default value, the solution space of the coupling parameter values for nonlinear firing outputs was drastically reduced by approximately 80%. As a result, the model tended to fire only in a linear mode at the majority of dendritic PIC sites. The same results were obtained when all parameters for the PIC activation simultaneously changed only by approximately ±10%. Our results suggest the democratization effect of neuromodulation: the neuromodulation by the brainstem systems may play a role in switching the motoneurons with PICs at different dendritic locations to a similar mode of firing by reducing the effect of the dendritic location of PICs on the firing behavior.
Spinal motoneurons have large, highly branched dendrites and voltage-gated ion channels that generate strong persistent inward currents (PICs) (Schwindt and Crill, 1980). Over the past 30 years, the impact of PICs on the firing output of the motoneurons has been extensively investigated in various species, including turtles (Hounsgaard and Kiehn, 1985, 1989), rats (Bennett et al., 2001; Li and Bennett, 2003), mice (Carlin et al., 2000; Meehan et al., 2010) and cats (Lee and Heckman, 1998, 1999). There has been a consensus in the motoneuron physiology community that in the presence of monoamines (i.e., norepinephrine and serotonin), the activation of the L-type Ca2+ PIC channels is facilitated, producing a long-lasting membrane depolarization (i.e., plateau potential) (reviewed in Powers and Binder, 2001; Heckman et al., 2008).
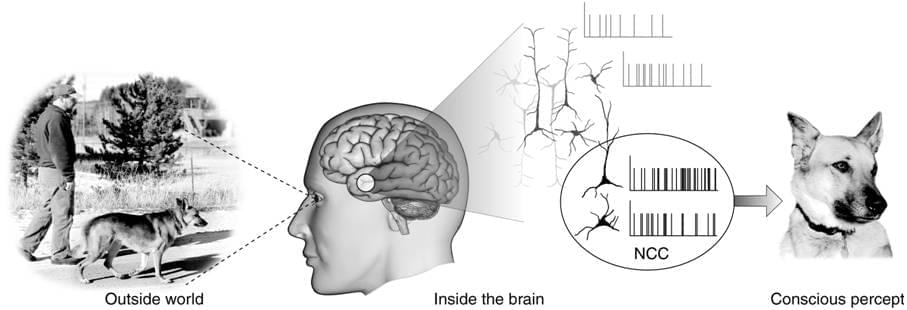
Can we in principle ever deduce the mental from the physical?
Christopher Devlin Brown and David Papineau have a new paper out in the Journal of Consciousness Studies titled: Illusionism and A Posteriori Physicalism; No Fact of the Matter. (Note: the link is to a free version.) As the title makes clear, the overall gist is that the difference between illusionism and a posteriori physicalism amounts to a definitional dispute.
A quick primer. Illusionism is the stance that consciousness exists, but only in the sense of functional capabilities such as modeling the self in its environment, attention, learning, episodic memory, self monitoring, etc. What’s thought to be illusory is phenomenal consciousness, the “what it’s like” nature of subjective experience, but particularly in the strong sense as something distinct from functional capabilities, and with properties, such as fundamental subjectivity, that imply it’s non-physical.
Susan Blackmore’s Consciousness: A Very Short Introduction may have been the first book I read on consciousness many years ago. Recent conversations rekindled my interest in her views.
I’m pretty sure her discussion of consciousness as an illusion was the first time I had encountered that idea. Strong illusionists such as Keith Frankish and Daniel Dennett generally take the stance that phenomenal consciousness doesn’t exist. Blackmore’s illusionism seems like a weaker form, that consciousness exists but isn’t what it seems. And by “consciousness” she stipulates in one of her books that she usually means phenomenal consciousness.
Of course, the difference between a strong and weak illusionist can be seen as mostly definitional. Strong illusionists generally take “phenomenal consciousness” to refer to the metaphysically intrinsic, private, ineffable, and incorrigible concept discussed by Nagel, Chalmers, and other non-physicalists, one that is ontologically separate from access (functional) consciousness. A weak illusionist sees this version as illusory, but is more willing to just consider the illusion itself a reconstructed version of “phenomenal”
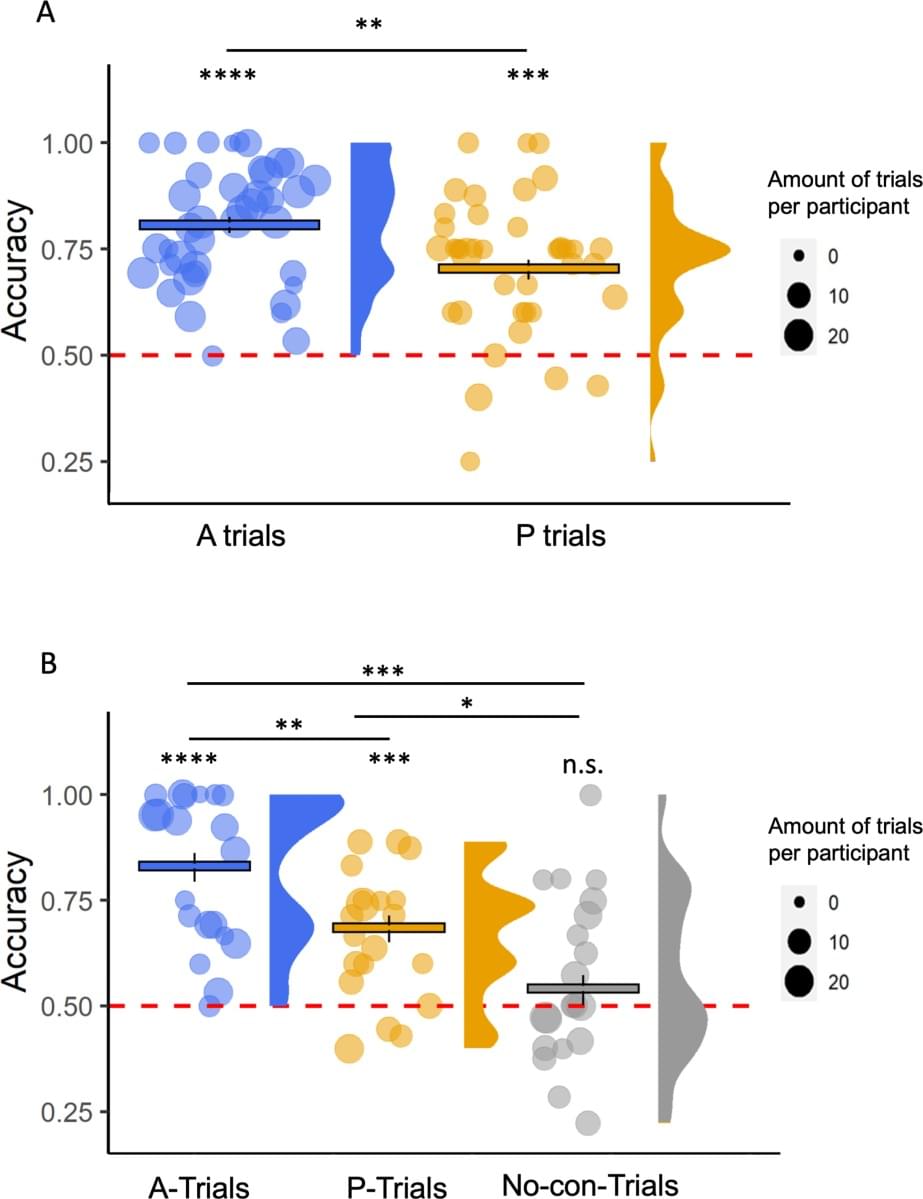
On Twitter, the Neuroskeptic shared a new paper, in which an Israeli team claims to have demonstrated phenomenal consciousness without access consciousness: Experiencing without knowing? Empirical evidence for phenomenal consciousness without access.
A quick reminder. In the 1990s Ned Block famously made a distinction between phenomenal consciousness (p-consciousness) and access consciousness (a-consciousness). P-consciousness is conceptualized as raw experience, qualia, the “what it’s like” nature of consciousness. A-consciousness is accessing that information for use in memory, reasoning, verbal report, or control of behavior.
The paper notes that this distinction is controversial. While widely accepted in many corners of consciousness studies, it’s been challenged by others, notably illusionists. The paper cites Daniel Dennett and Michael Cohen in particular as challenging the possibility of gathering data about p-consciousness, since any data gathered has to come through a-consciousness. It cites their 2011 paper, and a couple of others, as providing criteria for establishing p-consciousness.

Before I begin, I would like to state that the topics discussed here in reference to Quantum Mechanics represent my own understanding of the science. These ideas may or may not fall in with those who have achieved accreditation for their own understanding of the subject matter. The observations of the author are derived from extensive study of the science from a variety of materials, including audio and visual lectures. It is my hope that the understanding presented here will offer a medium of sorts for those concerned with preservation of personality.

In the last thread, someone asked what exactly is it about consciousness that illusionists say is illusory?
One quick answer is that for illusionists, the properties people see in experience that incline us to think that consciousness is a metaphysically hard problem, are what’s illusory. In weak illusionism, the properties aren’t what they seem. In the strong version, which is usually what “illusionism” refers to, they don’t exist at all. But what exactly are these properties?
I’m a functionalist, someone who sees conscious experiences, and mental states overall, as more about what they do, the causal roles they play, than about any particular substance or constitution. It’s a view that I think provides a necessary explanatory layer between the mental and the physical, and so sees no barrier in principle to a full understanding of the relationship between them.
I’m pretty much a subscriber to the computational theory of mind (broadly speaking), which holds that the mind is information in the brain. If this theory of mind is accurate, then there should be no barrier to someday uploading a copy of our mind into a computer, providing we can find a way to record it.
This is, of course, a controversial notion. There are many people who swear that uploading will never be accomplished. They list a lot of reasons, from the fact that the mind is inextricably entangled with the workings of the body, to the impossibility of ever making a fully accurate representation of the brain, to religious beliefs about mind / body dualism (which you won’t see me address in this post).
Regarding the notions about the mind being tangled with the body, I suspect the people who express these sentiments are underestimating what our ability will eventually be to virtualize these kinds of mechanisms. Sure, our mental states are tied to things like hormones, blood sugar level, the state of our gut, and many other body parameters. But many of these parameters are driven by the brain. And I don’t really see any reason why we wouldn’t eventually be able to simulate its effects on a virtual brain.
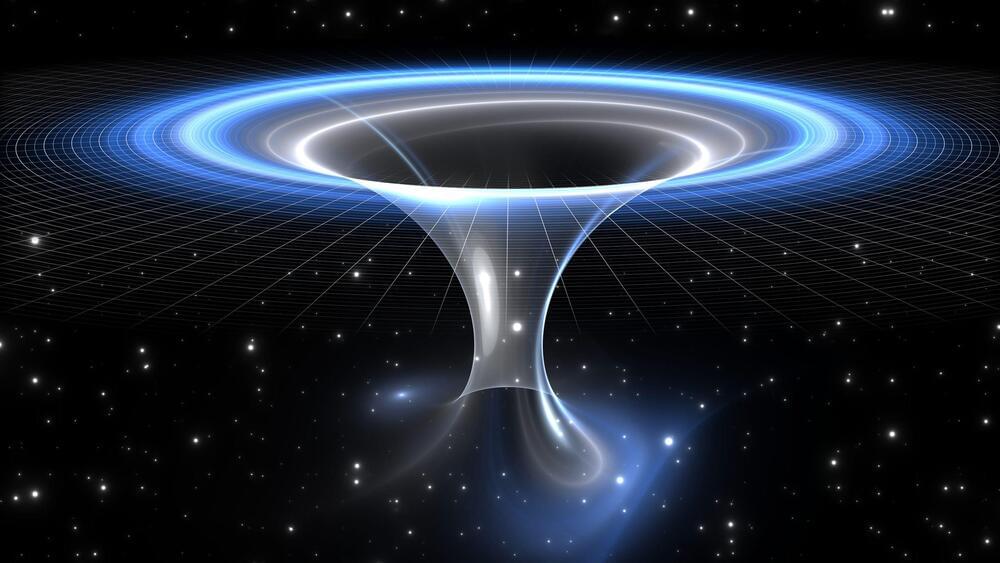
Some scientists believe black holes might be wormholes, offering shortcuts through space and time
A team of physicists from Sofia University in Bulgaria has proposed a fascinating theory that wormholes, hypothetical tunnels linking different parts of the universe, could be hiding in plain sight. These wormholes may resemble black holes so closely that current technology cannot distinguish between the two, according to a new study reported by New Scientist.