A new study examines the possibility of consciousness in artificial systems, focusing on ruling out scenarios where AI appears conscious without actually being so.


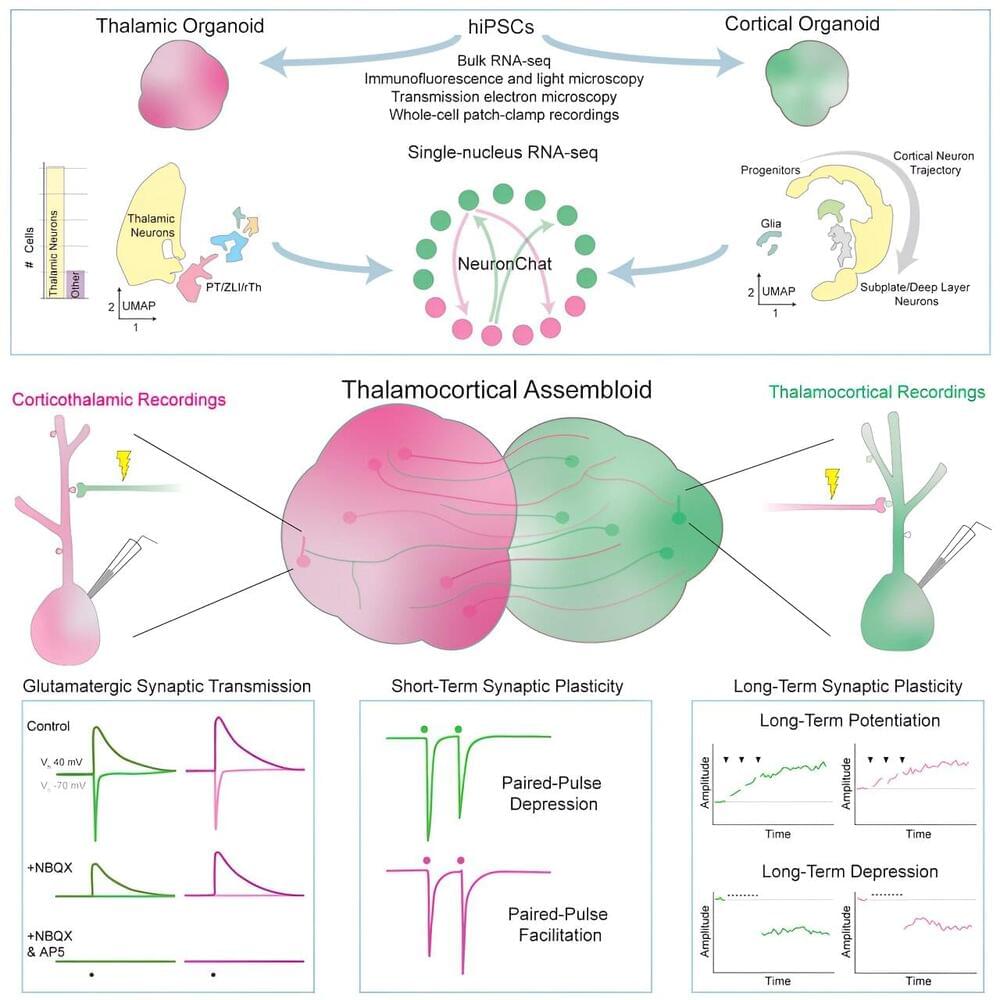
The ability to study human neurological systems depends on having viable, accurate models of brain function. St. Jude researchers have now created a model for such research by combining thalamic cells and cortical cells derived from human induced pluripotent stem cells.
The thalamocortical system mediates multiple sensory and cognitive processes, such as perception, learning and memory. The researchers developed a model of a primitive human thalamocortical system by maintaining thalamic and cortical cell masses known as organoids in close proximity in a culture dish.
In this model, the neurons in both organoids develop and grow long-ranging processes (axons) that extend to the opposite organoid and form functional connections (synapses). The researchers determined that when these synapses are stimulated, they undergo long-term strengthening and weakening of their electrical signals, which is the hallmark of synaptic plasticity, a process that underlies certain forms of learning and memory.


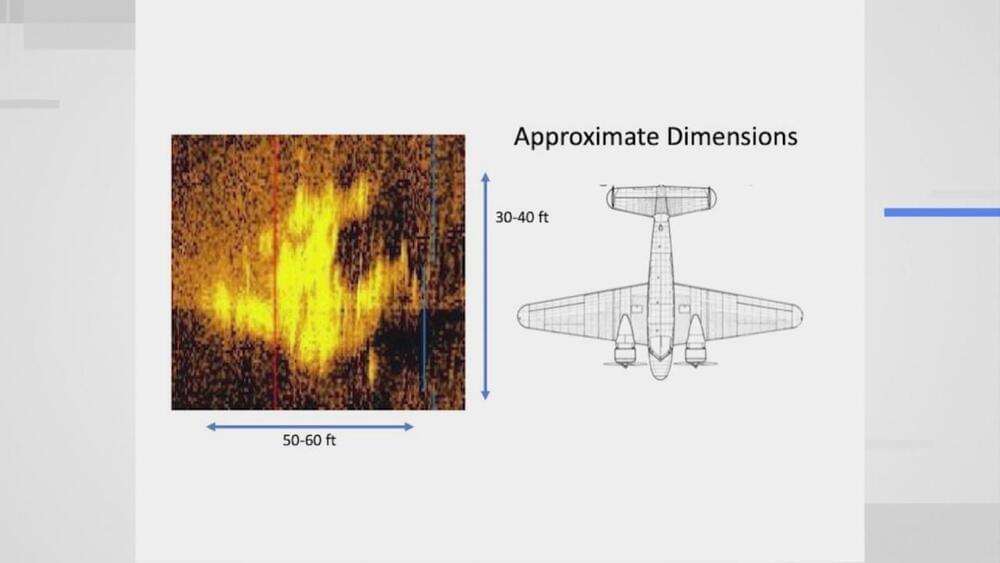
ATCHISON, Kan. (WDAF) — It’s one of the greatest unsolved mysteries ever, but we might now be on the verge of discovering what happened to Amelia Earhart’s plane.
Earhart was born and raised in Atchison, Kansas, and her love for planes and flying drove her to break barriers for female pilots.
On June 1, 1937, she and navigator Fred Noonan made an attempt to fly around the world in a Lockheed Electra 10-E plane, but somewhere over the Pacific, they lost radio contact and were never heard from again.
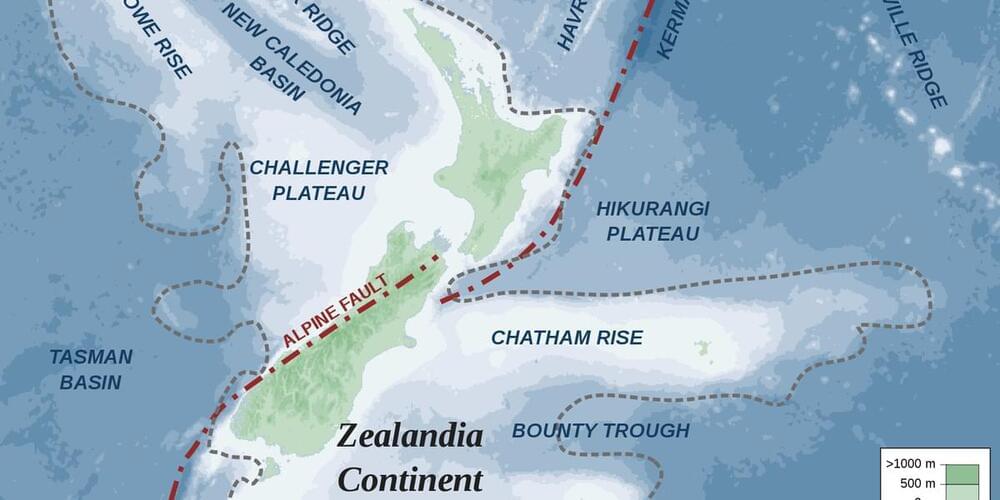
You might think you know all the continents, but what about Zealandia?
In 2017, a previously unknown contingent of the shores of New Zealand was discovered — making headlines globally.
Zealandia, known as Te Riu-a-Māui in the Māori language, covers more than 5 million square kilometres, making it twice the size of the subcontinent of India.
This remarkable miniature rotorcraft is so lightweight and efficient that it can lift its own mass given nothing but sunlight. The entire thing weighs about as much as four paperclips, and it can fly all day if the sun’s shining.
Researchers at China’s Beihang University and the Center of Advanced Aero-Engine, have unveiled CouloumbFly, a palm-sized miniature rotorcraft that weighs just 4.21 g (0.15 oz) – yet still boasts a rotor diameter of 20 cm (7.9 in), making it around 600 times lighter than any other comparable small solar-powered drone.
In tethered testing under natural sunlight conditions, CouloumbFly got itself airborne within a second and managed an hour of flight without power diminishing, before a mechanical failure brought it back down. Not much of a big deal if it was a glide-capable winged drone – but this is a miniature helicopter that’s entirely responsible for generating its own lift, and managing that on solar energy alone is an extraordinary feat.
Researchers administered an injection of an anti-IL-11 antibody to 75-week-old mice, neutralizing the harmful effects of IL-11.

Astronomers uncovered that a well-known X-ray binary, whose exact nature has been a mystery to scientists until now, is actually a hidden ultraluminous X-ray source. X-ray binaries are intriguing systems consisting of two celestial bodies: a normal star and a compact, dead object such as a black hole or a neutron star that sucks material from its stellar companion. A few hundred such sources have been identified thus far in our Galaxy. When it comes to the most powerful phenomena in the Universe, the release of gravitational energy in X-ray binary systems stands out as a highly efficient process.
Among the first X-ray binary systems discovered in the cosmos is the system Cygnus X-3. Since the early 1970s, this binary system was noted for its ability to briefly emerge as one of the most intense radio sources, yet in a few days it dims or vanishes altogether.
This peculiar characteristic spurred early efforts, coordinated by telephone calls, to unite astronomical observations across the globe.
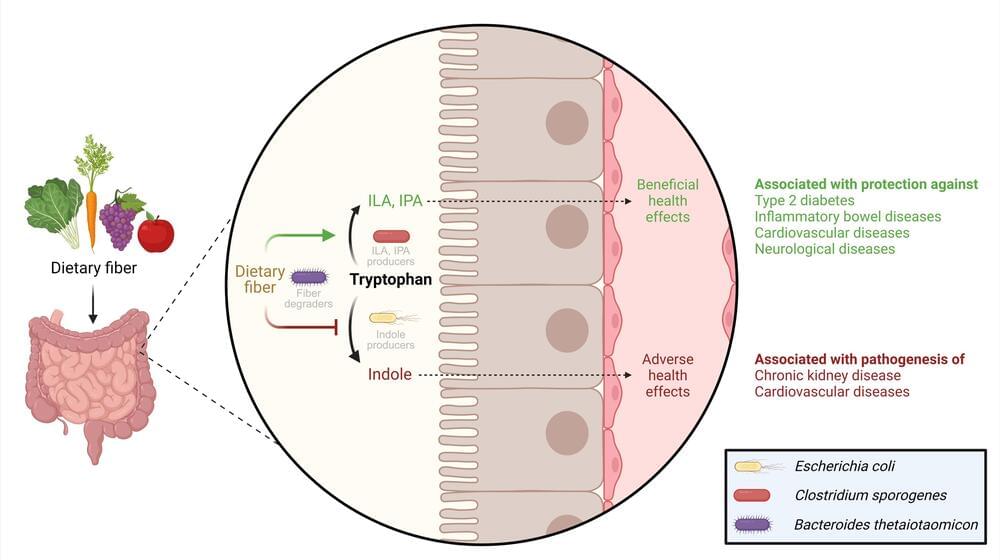
We get healthy dietary fiber from consuming fruits, vegetables, and whole grains. But why is fiber so good for us? A team of researchers has discovered that dietary fiber plays a crucial role in determining the balance between the production of healthy and harmful substances by influencing the behavior of bacteria in the colon.
Dietary fiber benefits our health, and scientists from DTU National Food Institute and the Department of Nutrition, Exercise and Sports at the University of Copenhagen have now uncovered an essential part of why this is the case. Different types of bacteria inside our colon compete to utilize an essential amino acid called tryptophan. This competition may lead to either good or bad outcomes for our health.
The research, published in the journal Nature Microbiology, reveals that when we eat a lot of dietary fiber, gut bacteria help turn tryptophan into healthy substances. But if we don’t eat enough fiber, tryptophan can be converted into harmful compounds by our gut bacteria.