The red giant star will explode, but it’s unknown when.


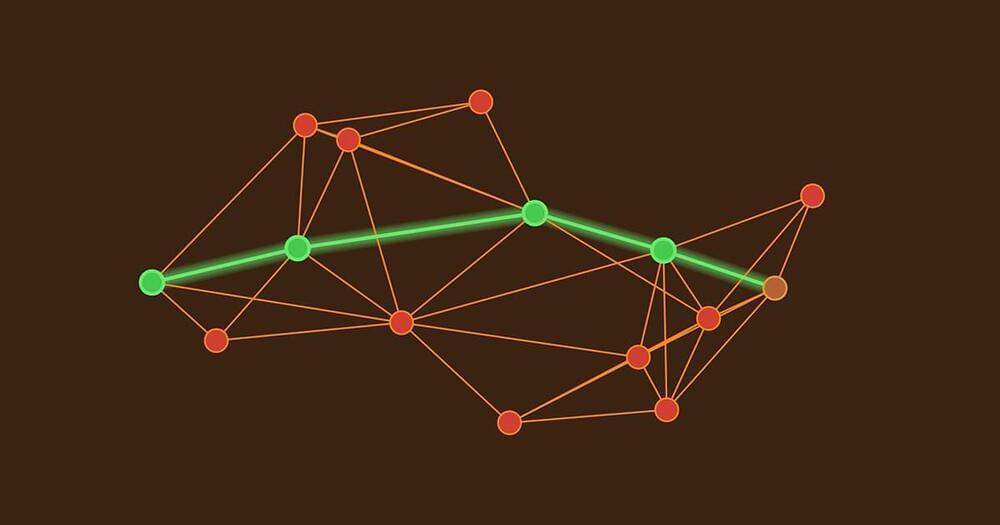
A new proof shows that an upgraded version of the 70-year-old Dijkstra’s algorithm reigns supreme: It finds the most efficient pathways through any graph.
It doesn’t just tell you the fastest route to one destination.
In an interview toward the end of his life, Dijkstra credited his algorithm’s enduring appeal in part to its unusual origin story. “Without pencil and paper you are almost forced to avoid all avoidable complexities,” he said.
Dijkstra’s algorithm doesn’t just tell you the fastest route to one destination. Instead, it gives you an ordered list of travel times from your current location to every other point that you might want to visit — a solution to what researchers call the single-source shortest-paths problem. The algorithm works in an abstracted road map called a graph: a network of interconnected points (called vertices) in which the links between vertices are labeled with numbers (called weights). These weights might represent the time required to traverse each road in a network, and they can change depending on traffic patterns. The larger a weight, the longer it takes to traverse that path.
To get a sense of Dijkstra’s algorithm, imagine yourself wandering through a graph, writing down the travel time from your starting point to each new vertex on a piece of scratch paper. Whenever you have a choice about which direction to explore next, head toward the closest vertex you haven’t visited yet. If you discover a faster route to any vertex, jot down the new time and cross out the old one. When you’re sure that you’ve found the fastest path, move the travel time from your notes to a separate, more presentable list.
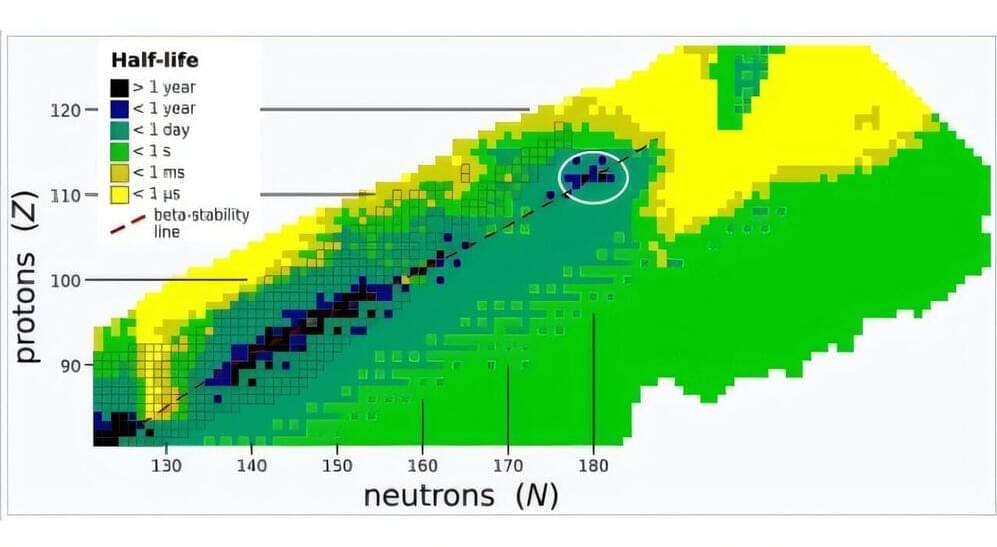
What is the heaviest element in the universe? Are there infinitely many elements? Where and how could superheavy elements be created naturally?
The heaviest abundant element known to exist is uranium, with 92 protons (the atomic number “Z”). But scientists have succeeded in synthesizing superheavy elements up to oganesson, with a Z of 118. Immediately before it are livermorium, with 116 protons and tennessine, which has 117.
All have short half-lives—the amount of time for half of an assembly of the element’s atoms to decay—usually less than a second and some as short as a microsecond. Creating and detecting such elements is not easy and requires powerful particle accelerators and elaborate measurements.
Haven’t heard from Bill Andrews in awhile.
BiOptimizers Magnesium Breakthrough 10% with code Modern10 https://bioptimizers.com/modern. This video brought to you by BiOptimizers.
Here we talk with Dr Bill Andrews all about telomeres, why they are on the critical path of aging and finding a way to lengthen them is required in an complete longevity solution.
Some links are affiliate links so we will earn a commission when they are used to purchase products.
If you would like to support our channel please consider joining our patreon / modernhealthspan.
Stemregen 15% discount Code MODERN https://tinyurl.com/45z968yr (Only available in the US)
Renue By Science 10% discount code MHS: https://tinyurl.com/bdew4bfs.
NMN Powder https://tinyurl.com/syc7rwkh.
DoNotAge 10% discount code MHS https://tinyurl.com/6dbvhv87
NMN https://tinyurl.com/wyzj2f3d CaAKG https://tinyurl.com/2h79stt2
Wellness Extract 10% discount Code MODERNWE Geranylgeraniol Essential http://wellnessextract.com/RICHARDWE Delta Gold Vit E
n1o1 Nitric Oxide 10% discount with code Modern https://tinyurl.com/3esakm4s.
n1o1 Nitric Oxide Lozenges https://tinyurl.com/yh4rrtht.
Age-Defiance Face Cream https://tinyurl.com/4zr959zh.
OmegaQuant 5% discount Code MODERN https://omegaquant.com/shop/
Bulletproof 15% off with coupon code: HEALTHSPAN15: https://tinyurl.com/4npjk5vp.
Inner Fuel Gut support https://bulletproof.fdf2.net/PyDKDM
Omega-3 Krill Oil https://bulletproof.fdf2.net/xkdxmy.
Pendulum Akkermansia pendulumtherapeutics.sjv.io/baoQVg.
Metabolic Daily https://pendulumtherapeutics.sjv.io/N…
Nuchido Time+ 20% discount of first purchase with code MODERN20 https://nuchido.com/MODERN
OneSkin 15% Discount: Code MODERN https://tinyurl.com/3t6tevj8 OS-01 Face Topical Supplement https://tinyurl.com/29c8wrr2
Neurohacker Qualia Senolytic https://tinyurl.com/22t9thrn.
TruDiagnostics 12% Discount Code MODERN TruAGE PACE https://trudiagnostic.pxf.io/oqYVMY
☕If you would like to support our channel, we’d love a coffee…thank you! https://www.buymeacoffee.com/mhealthspan.
⏲️Chapters.
00:00 Why telomeres.
07:10 Telomeres and aging.
14:36 Telomerase in stem cells.
20:15 BiOptimizers.
21:44 Telomere shortening.
23:25 Measuring telomere length.
25:30 Biological age.
30:30 Slowing telomere shortening.
39:30 Supplements to help.
41:30 Sierra Science Chemicals \& Gene therapy.
49:30 How is telomerase repressed.
56:10 Liz Parrish \& Gene therapy.
1:01:40 Gene therapy delivery.
1:09:20 Telomerase \& cancer.
1:17:00 Why aging.
1:20:30 Telomeres \& senescence.
1:24:30 More information.
🌐Links in this video.

It is well-established that chronic MSK pain is the key factor for physical disability in the adult population. 19 The World Health Organization (WHO) estimates that 20–33% (over 1.71 billion individuals) of the global population suffers from chronic MSK pain. 20 This type of disorder is characterized by acute or chronic pain in MSK structures, which involve muscles, tendons, ligaments, bones, and nerves. 21 The most common conditions responsible for visits to a physician’s office are OA, rheumatoid arthritis, myofascial pain syndrome (MPS), and low back and neck pain. 22 Less common incidents are generally accompanying with injuries like of tendon sprains, ligament tears, muscle tears, fractures, and similar damage during sports. 20
If left untreated, these conditions progressively increase suffering, disability, and drug consumption, which subsequently diminish an individual’s quality of life. 23 This also translates to a main community health problem due to significant high expenses for health-care systems and insurance for disability. Advanced age may remain the top variable associated with the increased risk of musculoskeletal disorders (MSDs) and MSK pain; however, these conditions may still unfold at any given age for various reasons. Therefore, every individual is at risk of experiencing MSK pain throughout an entire lifetime. 24 Acute pain can become chronic due to numerous factors. The level of intensity, site, and time of noxious stimuli are dictated by the interplay between mechanical, chemical, and thermal receptors and immune cells. 25 Under standard conditions, noxious stimuli and painful sensations gradually decrease with the progression of healing.

Gridstor, a US-based developer and operator of grid-scale battery storage systems, has kicked off construction of its first project in the Texas ERCOT market.
The developer said last week (17 October) that construction is underway on the Hidden Lakes Reliability Project 220MW/440MWh standalone battery energy storage system (BESS) in Texas’ Galveston County.

In a major jump into the era of eVTOL air taxis and multicopter cargo drones, the US FAA has issued new regulations that introduce the first new aircraft category, called “power-lift” aircraft, since modern helicopters were introduced in the 1940s.
According to the FAA and the International Civil Aviation Organization (ICAO), a power-lift aircraft is “a heavier-than-air aircraft capable of vertical take-off, vertical landing, and low-speed flight, which depends principally on engine-driven lift devices or engine thrust for the lift during these flight regimes and on non-rotating aerofoils for lift during horizontal flight.”
Essentially, this means aircraft that combine the characteristics of both fixed-wing planes and helicopters. In other words, they can take off, hover, and land like helicopters, yet act like fixed-winged craft in horizontal flight. As of now, these include convertiplanes, tilt-rotors, tilt-wings, rotor-wings, tail-sitters, and VSTOL aircraft like the Harrier and the F-35B Lighting II that use vector thrust, lift jets, or lift fans for vertical flight.


Recent findings indicate that gamma-ray emissions from thunderstorms are far more complex and dynamic than previously understood, thanks to observations of new phenomena like Flickering Gamma-Ray Flashes (FGFs).
These flashes, alongside in-depth studies on tropical thundercloud emissions, suggest that our understanding of atmospheric electricity is evolving. This is supported by extensive fieldwork during the ALOFT campaign, which gathered unprecedented data over the Gulf of Mexico and surrounding areas.
Thunderstorm Gamma-Ray Emissions