In order to figure out how something came from nothing, we first need to explore the different types of nothing.


In order to figure out how something came from nothing, we first need to explore the different types of nothing.

With their slender tails, human sperm propel themselves through viscous fluids, seemingly in defiance of Newton’s third law of motion, according to a recent study that characterizes the motion of these sex cells and single-celled algae.
Kenta Ishimoto, a mathematical scientist at Kyoto University, and colleagues investigated these non-reciprocal interactions in sperm and other microscopic biological swimmers, to figure out how they slither through substances that should, in theory, resist their movement.
When Newton conceived his now-famed laws of motion in 1686, he sought to explain the relationship between a physical object and the forces acting upon it with a few neat principles that, it turns out, don’t necessarily apply to microscopic cells wriggling through sticky fluids.

Until now, only a small fraction of meteorites that land on Earth had been firmly linked back to their parent body out in space – but a set of new studies has just given us compelling origin stories for more than 90 percent of meteorites today.
Past analyses of meteorites striking our planet today suggest some kind of shared origin; they’re made from very similar materials and have been baked by cosmic radiation for a suspiciously short amount of time, hinting at a relatively recent break-up from shared parent bodies.
The teams behind three new published papers used a combination of super-detailed telescope observations and computer modeling simulations to compare asteroids out in space with meteorites recovered on Earth, matching up rock types and orbital paths between the two.

Instead of the old-fashioned hammer and chisel, a 13-foot zinc alloy arm with a spinning, diamond-crusted finger is now used by some to cut marble. Robotor CEO Giacomo Massari says it’s ten times faster.
A fleet of marble-sculpting robots is carving out the future of the art world. It’s a move some artists see as cheating, but others are embracing the change.
Magnetotherapy has been receiving increased attention as an attractive strategy for modulating cell physiology directly at the site of injury, thereby providing the medical community with a safe and non-invasive therapy.
Pesqueira, T., Costa-Almeida, R. & Gomes, M.E. Sci Rep 7, 10,948 (2017). https://doi.org/10.1038/s41598-017-11253-6
Mimicking how plants convert sunlight into energy has long been a dream for scientists aiming to create renewable energy solutions. Artificial photosynthesis is a process that seeks to replicate nature’s method, using sunlight to drive chemical reactions that generate clean energy. However, creating synthetic systems that work as organically as natural photosynthesis has been a significant challenge until now.
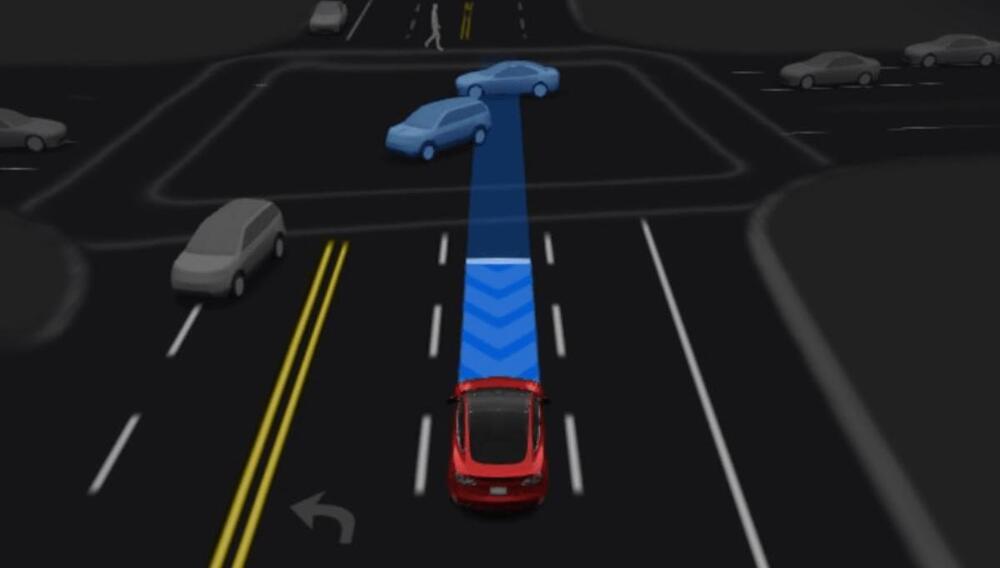
Tesla began rolling out a significant update to its Full Self-Driving (FSD) software on Saturday, shifting the city-streets driving system to a single, end-to-end neural network model in FSD version 12.5.6.3.
Last week, Tesla CEO Elon Musk said the company’s FSD technology “is now almost entirely AI.” In early October, Musk had stated that FSD “will soon exceed 10,000 miles between critical interventions, which is a year of driving for most people.”


After thousands of years as a highly valuable commodity, silk continues to surprise. Now it may help usher in a whole new direction for microelectronics and computing.
While silk protein has been deployed in designer electronics, its use is currently limited in part because silk fibers are a messy tangle of spaghetti-like strands.
Now, a research team led by scientists at the Department of Energy’s Pacific Northwest National Laboratory has tamed the tangle. They report in the journal Science Advances (“Two-dimensional silk”) that they have achieved a uniform two-dimensional (2D) layer of silk protein fragments, or “fibroins,” on graphene, a carbon-based material useful for its excellent electrical conductivity.

A 9th grader from Snellville, Georgia, has won the 3M Young Scientist Challenge, after inventing a handheld device designed to detect pesticide residues on produce.
Sirish Subash set himself apart with his AI-based sensor to win the grand prize of $25,000 cash and the prestigious title of “America’s Top Young Scientist.”
Like most inventors, Sirish was intrigued with curiosity and a simple question. His mother always insisted that he wash the fruit before eating it, and the boy wondered if the preventative action actually did any good.