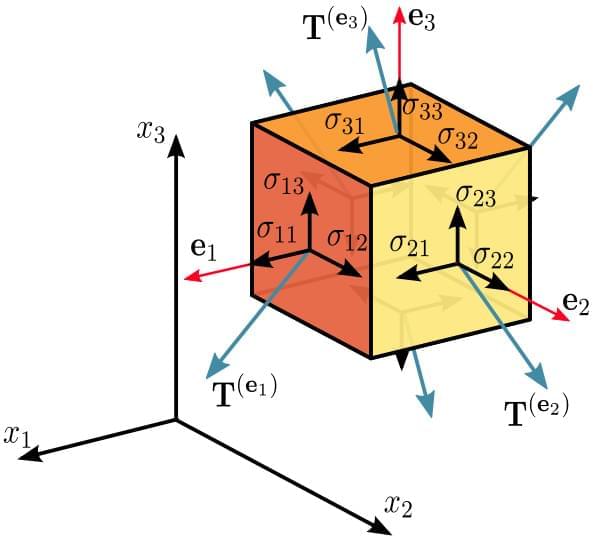
“the Cauchy stress tensor completely defines the state of stress at a point inside a material in the deformed state”
The Cauchy Stress Tensor.
Imagine you’re holding a rubber band in your hands and stretching it.
The SI base units of both stress tensor and traction vector are newton per square metre (N/m2) or pascal (Pa), corresponding to the stress scalar. The unit vector is dimensionless.
The Cauchy stress tensor obeys the tensor transformation law under a change in the system of coordinates. A graphical representation of this transformation law is the Mohr’s circle for stress.
The Cauchy stress tensor is used for stress analysis of material bodies experiencing small deformations: it is a central concept in the linear theory of elasticity. For large deformations, also called finite deformations, other measures of stress are required, such as the Piola–Kirchhoff stress tensor, the Biot stress tensor, and the Kirchhoff stress tensor.