DeepMind boss Demis Hassabis suggests Google will spend more than $100 billion on AI development as Alphabet battles rivals for supremacy.
Check out the math & physics courses that I mentioned (many of which are free!) and support this channel by going to https://brilliant.org/Sabine/ where you can create your Brilliant account. The first 200 will get 20% off the annual premium subscription. In the diagram at 4 minutes 30 seconds, the labels for h_1 and h_2 are mixed up. Sorry about that! Subscribe to my weekly science newsletter: https://sabinehossenfelder.com/ Everything I am talking about here is standard material of undergrad physics textbooks. My personal favorite is good old Goldstein https://en.wikipedia.org/wiki/Classic… On variational principles more specifically I quite like this textbook: https://link.springer.com/book/10.100… You can support me on Patreon: / sabine 0:00 Intro 0:45 Optimization 1:35 Shortest Path 3:20 Least Time 5:36 Least Action 10:27 Quantum Mechanics 11:53 Sponsor Message.
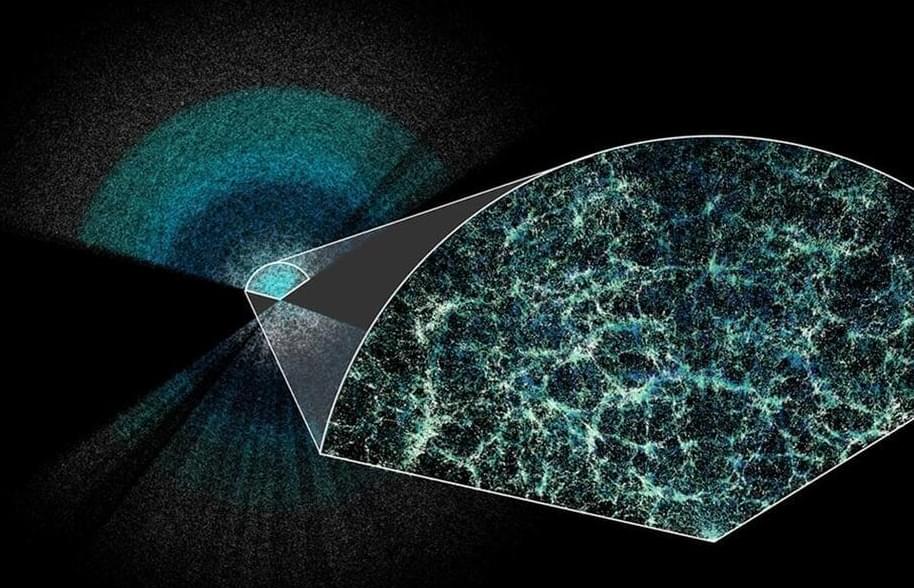
I found this on NewsBreak: Dark energy could be getting weaker, suggesting the universe will end in a ‘Big Crunch’
The first year of Dark Energy Spectroscopic Instrument (DESI) data seems to show that dark energy is weakening over time, possibly the biggest cosmological discovery for 25 years.
I found this on NewsBreak: Unraveling Dark Energy and Cosmic Expansion With an 11-Ton Time Machine.
I found this on NewsBreak: The spinal cord can learn and remember independently of the brain.
How Food Changes Your Brain
Posted in food, neuroscience
I found this on NewsBreak: Scientists finally make ‘goldene’, potentially breakthrough new material.
Researchers have managed to create “goldene”, an incredibly thin version of gold.
The work follows the successful production of graphene, which is made out of a single layer graphite atoms. That has been hailed as a miracle material: it is astonishingly strong, and much better at conducting heat and electricity than copper.
Goldene is built on the same principle, with researchers spreading out gold so it is just one atom layer thick. And, similar to graphene, scientists say that the process gives it a variety of new properties that could lead to major breakthroughs.
I found this on NewsBreak: The big idea: are we about to discover a new force of nature?
Intriguingly, both disciplines are grappling with unexplained results that could be pointing to the existence of a new force of nature. If such a new force were to be confirmed, the implications for our understanding of the universe, its history and makeup would be profound.
There are four forces that we already know about. Gravity governs the grandest scales, marshalling the planets in their orbits and shaping the evolution of the universe as a whole. Electromagnetic force gives rise to a vast range of phenomena, from the magnetic field of the Earth to radio waves, visible light and X-rays, while also holding atoms, molecules and, by extension, the physical world together. Deep within the atomic nucleus, two further forces emerge: the vice-like “strong force”, which binds atomic nuclei, and the “weak force”, which among other things causes radioactive decay and enables the nuclear reactions that power the sun and the stars.
Studying these forces has transformed our understanding of nature and generated revolutionary new technologies. Work on electromagnetism in the 19th century gave us the electric dynamo and radio broadcasts, the discovery of the strong and weak forces in the 1930s led to nuclear energy and atomic bombs, while understanding gravity has made it possible to put astronauts on the moon and to develop GPS satellites that can tell us our location anywhere on Earth to within a few metres. Uncovering a fifth force would be one hell of a prize.