Ancient human genomes reconstructed from remains at a southern African rock shelter show remarkable genetic continuity over time.



Source: Nottingham Trent University.
Scientists have identified previously unreported genes which appear to play a key role in the muscle aging process. It is hoped that the findings from a Nottingham Trent University study could be used to help delay the impact of the aging process.
The study, which also involved Sweden’s Karolinska Institute, Karolinska University Hospital, and Anglia Ruskin University, is reported in the Journal of Cachexia, Sarcopenia and Muscle.
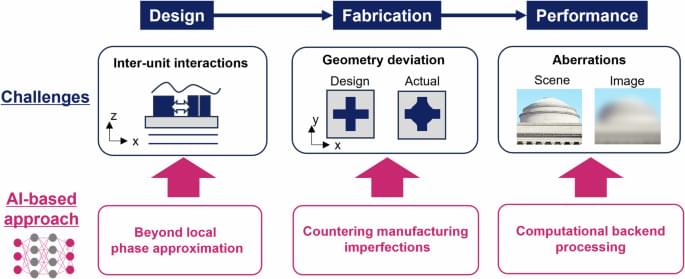
Optica l metasurfaces, planar artificial media capable of controlling light propagation, areioning from laboratory curiosity to commercial applications.
npj Nanophotonics volume 1, Article number: 36 (2024) Cite this article.

When I was a kid we had the anarchist cookbook.
But an artist and hacker found a way to trick ChatGPT to ignore its own guidelines and ethical responsibilities to produce instructions for making powerful explosives.
The hacker, who goes by Amadon, called his findings a “social engineering hack to completely break all the guardrails around ChatGPT’s output.” An explosives expert who reviewed the chatbot’s output told TechCrunch that the resulting instructions could be used to make a detonatable product and was too sensitive to be released.





The Internet Archive was breached again, this time on their Zendesk email support platform after repeated warnings that threat actors stole exposed GitLab authentication tokens.
Since last night, BleepingComputer has received numerous messages from people who received replies to their old Internet Archive removal requests, warning that the organization has been breached as they did not correctly rotate their stolen authentication tokens.
“It’s dispiriting to see that even after being made aware of the breach weeks ago, IA has still not done the due diligence of rotating many of the API keys that were exposed in their gitlab secrets,” reads an email from the threat actor.