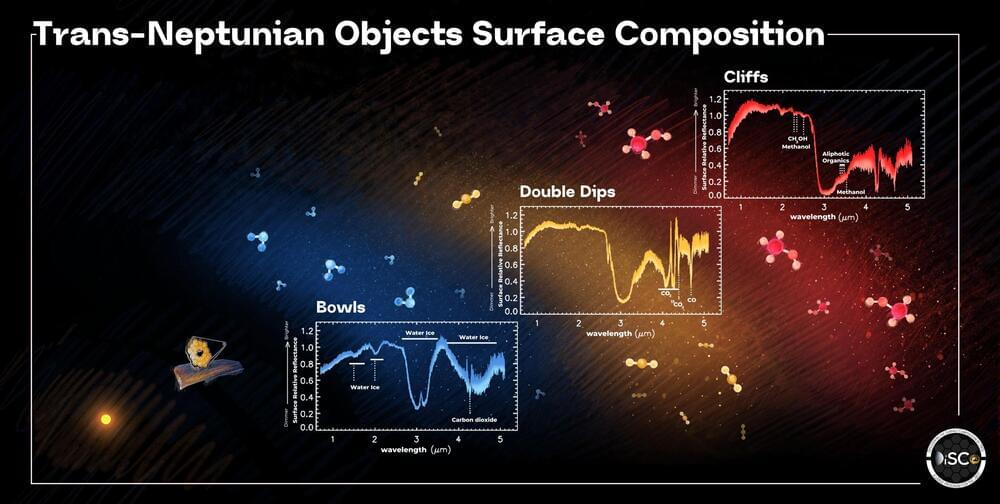
New studies led by researchers at the University of Central Florida offer for the first time a clearer picture of how the outer solar system formed and evolved based on analyses of trans-Neptunian objects (TNOs) and centaurs.
The findings, published today in Nature Astronomy, reveal the distribution of ices in the early solar system and how TNOs evolve when they travel inward into the region of the giant planets between Jupiter and Saturn, becoming centaurs.
TNOs are small bodies, or “planetesimals,” orbiting the sun beyond Pluto. They never accreted into planets, and serve as pristine time capsules, preserving crucial evidence of the molecular processes and planetary migrations that shaped the solar system billions of years ago. These solar system objects are like icy asteroids and have orbits comparable to or larger than Neptune’s orbit.