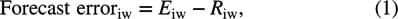Quantitative measures of bubble activity may improve medical therapies including drug delivery and tumor treatments.

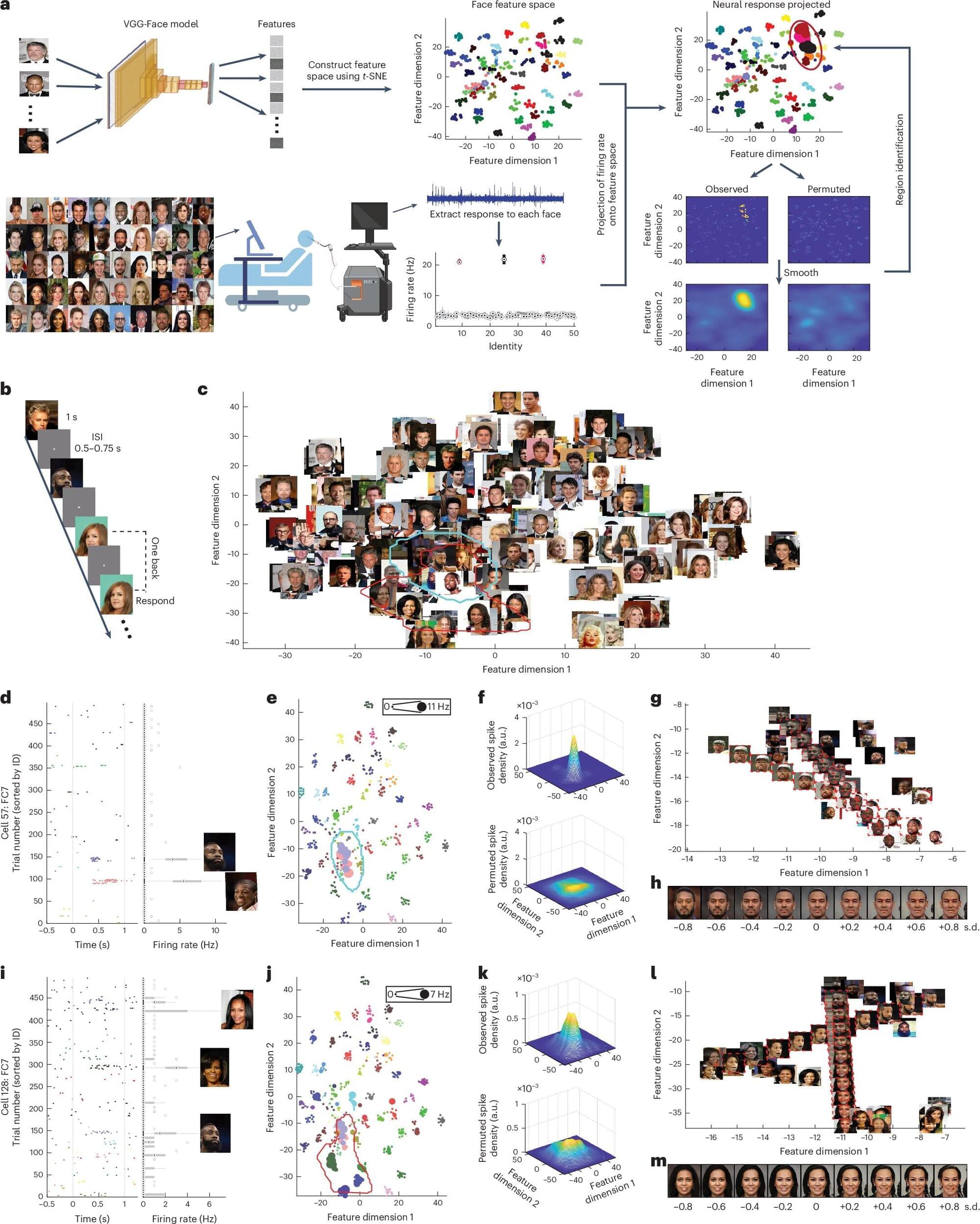
Humans are innately capable of recognizing other people they have seen before. This capability ultimately allows them to build meaningful social connections, develop their sense of identity, better cooperate with others, and identify individuals who could pose a risk to their safety.
Several past studies rooted in neuroscience, psychology and behavioral science have tried to shed light on the neural processes underlying the ability to encode other people’s identities. Most findings collected so far suggest that the identity of others is encoded by neurons in the amygdala and hippocampus, two brain regions known to support the processing of emotions and the encoding of memories, respectively.
Based on evidence collected in the past, researchers have concluded that neurons in these two brain regions respond in specific ways when we meet a person we are acquainted with, irrespective of visual features (i.e., how their face looks). A recent paper published in Nature Human Behaviour, however, suggests that this might not be the case, and that individual neurons in the amygdala encode and represent facial features, ultimately supporting the identification of others.
From enabling quantum supercomputers to securing communications and teleporting quantum states, entanglement is the thread weaving through all of quantum technology. What once struck Einstein as a paradox is today routinely observed and harnessed in labs – the “spooky action” has become a practical tool. We have learned that entanglement is not some esoteric fringe effect; it’s a concrete physical resource, much like energy or information, that can be exploited to do tasks that are otherwise impossible. Its special correlations allow quantum computers to perform massively parallel computations in a single wavefunction, allow cryptographers to detect eavesdroppers with absolute certainty, and allow quantum states to be transmitted without moving a physical carrier.
Yet, there is still much to master. Entangling a handful of qubits is easy; doing so with thousands or millions – while keeping them error-corrected – remains a grand challenge. As we push the number of entangled particles higher, we are essentially scaling up new forms of matter (entangled states) that have no counterpart in classical physics. In 2022, a 12-qubit entangled state might be a small quantum circuit; by 2035, we could be operating machines where 1,000 qubits are all entangled in complex ways, delivering computational feats far beyond today’s reach. On the communications front, nascent quantum networks are entangling nodes over city-scale distances, working toward a future quantum internet that could interconnect quantum computers or enable clock synchronization and sensing with unprecedented precision. Each improvement in generating high-quality entanglement over distance inches us closer to unhackable global communication links.
Entanglement also raises philosophical questions about the nature of reality – it blurs the boundary between “separate” objects and challenges our intuitions of locality. But from an engineer’s perspective, entanglement is also just another phenomenon to be tamed and utilized. The narrative of quantum technology is one of turning quantum quirks into quantum capabilities. Where classical engineers use wires and signals, quantum engineers use entanglement and superposition. It’s telling that entanglement is often called the “essence” or “cornerstone” of quantum mechanics – crack it, and you unlock a whole new paradigm of information processing.

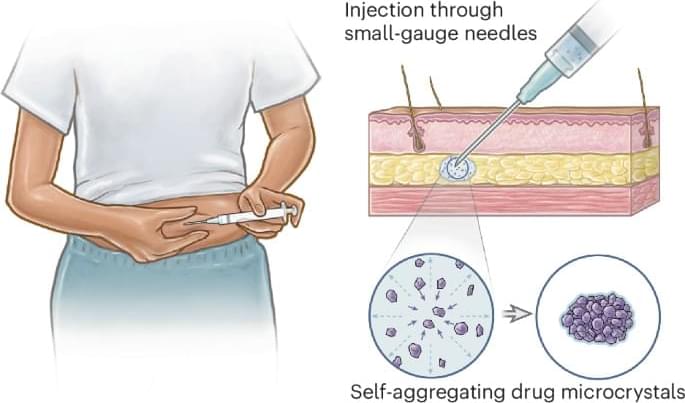
This study reports on self-aggregating injectable microcrystals for administering long-acting drug implants via low-profile needles, a key factor in patient adoption. Microcrystal self-aggregation is engineered through a solvent exchange process to form depots with minimal polymer excipient, demonstrating enhanced long-term release of a model contraceptive drug in rodents.



ImageimagePoorly energized mitochondria trap a subpopulation of mitochondrial precursor proteins in the intermembrane space. This article introduces ‘mitochondrial triage of precursor proteins’ (MitoTraP) as a mechanism that prevents the mistargeting of non-imported proteins to the nucleus and reduces proteotoxic effects.