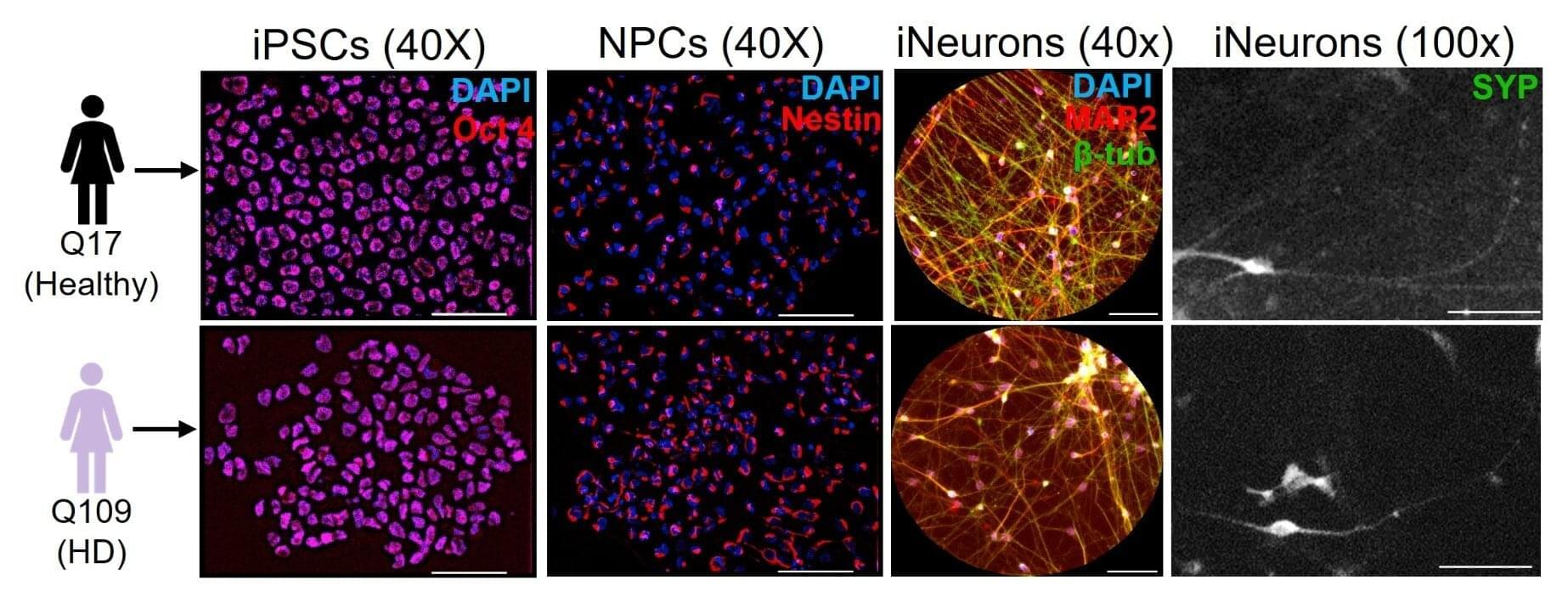
A decade ago, University at Buffalo researchers shed some light on an enduring neuroscience mystery: How exactly does a mutated huntingtin protein (HTT) cause Huntington’s disease?
They found that HTT is something like a traffic controller inside neurons, moving different cargo along neuronal highways called axons in concert with other proteins that are key for cellular function and survival. Reduce the amount of non-mutant HTT and you’ll create the neurological equivalent of traffic jams and roadblocks.
Now, the researchers have learned more about what can control the traffic-controlling HTT.