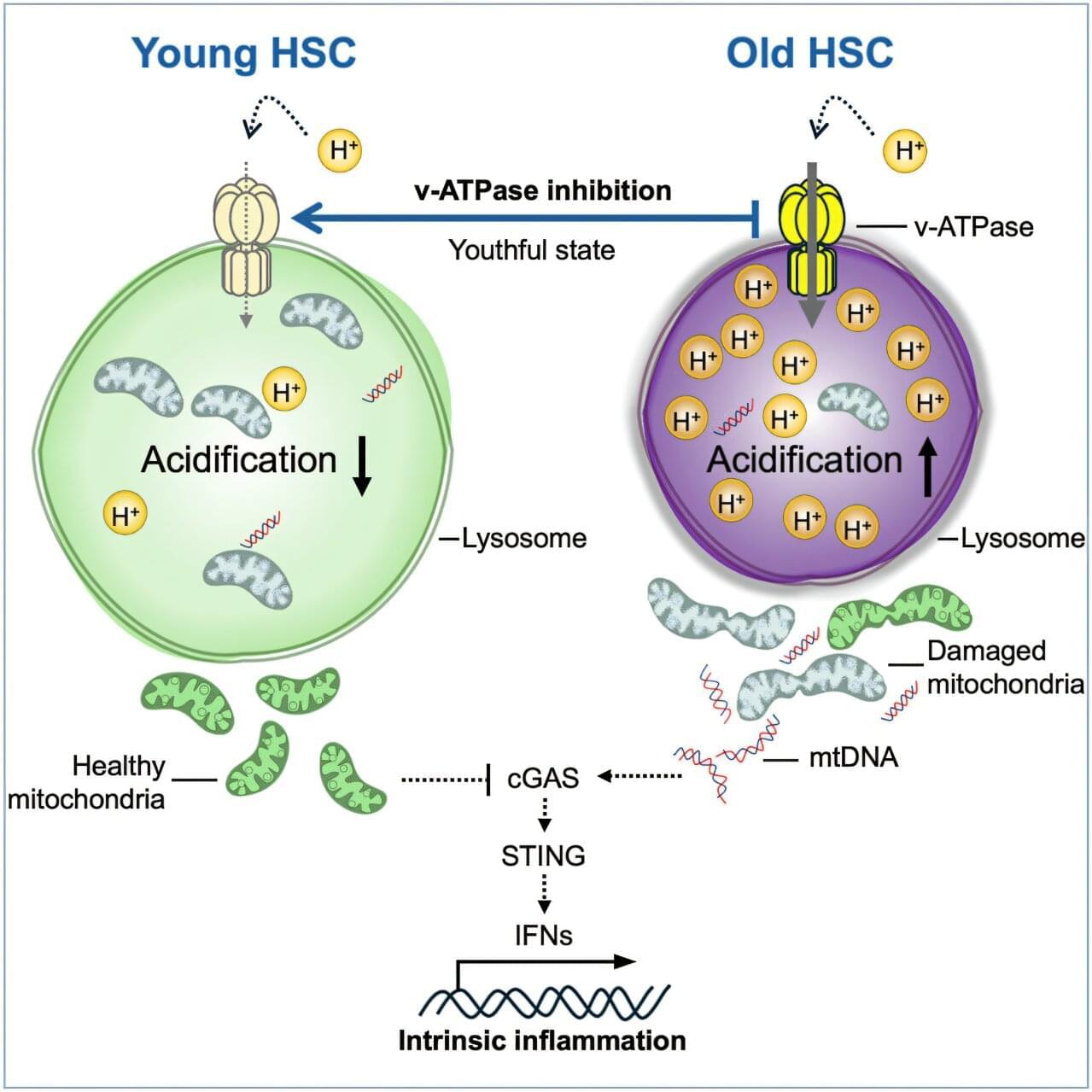
Amazon founder Jeff Bezos is stepping back into an active operating role in technology after four years, taking the co‑chief executive seat at a new artificial intelligence company called Project Prometheus. His move adds to the trend of tech billionaires emerging from semi‑retirement to participate directly in the AI boom reshaping Silicon Valley’s priorities.
Reportedly, Bezos will lead the startup alongside Vik Bajaj, a physicist‑chemist and former Google researcher who helped launch Verily, Alphabet’s life sciences unit. Project Prometheus has already raised about 6.2 billion dollars, including a substantial personal commitment from Bezos, placing it among the best‑funded early‑stage AI companies in the world.
Bezos has remained executive chair of Amazon and continued to back Blue Origin, his private space company, but Prometheus is his first formal operational role since he stepped down as Amazon’s CEO in 2021. The venture comes amid an intensifying global race to build advanced AI systems, as companies across the United States, Europe, and China pour money into research, data centres, and specialist talent.