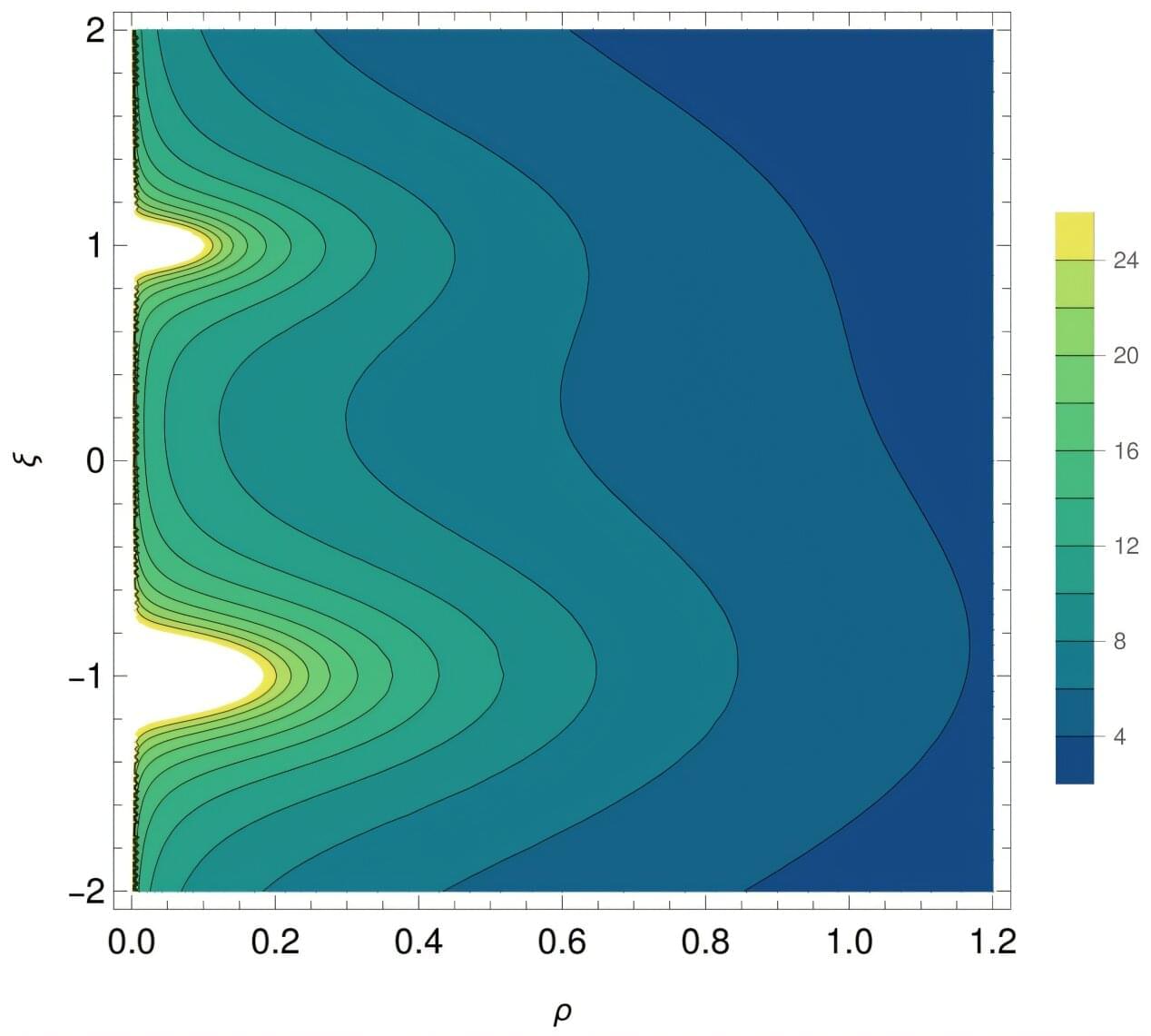
A team of physicists have discovered a new approach that redefines the conception of a black hole by mapping out their detailed structure, as shown in a research study recently published in Journal of High Energy Physics.
The study details new theoretical structures called “supermazes” that offer a more universal picture of black holes to the field of theoretical physics. Based in string theory, supermazes are pivotal to understanding the structure of black holes on a microscopic level.
“General relativity is a powerful theory for describing the large-scale structure of black holes, but it is a very, very blunt instrument for describing black-hole microstructure,” said Nicholas Warner, co-author of the study and professor of physics, astronomy and mathematics at the USC Dornsife College of Letters, Arts and Sciences. In a framework of theories extending beyond Einstein’s equations, supermazes provide a detailed portrait of the microscopic structure of brane black holes.