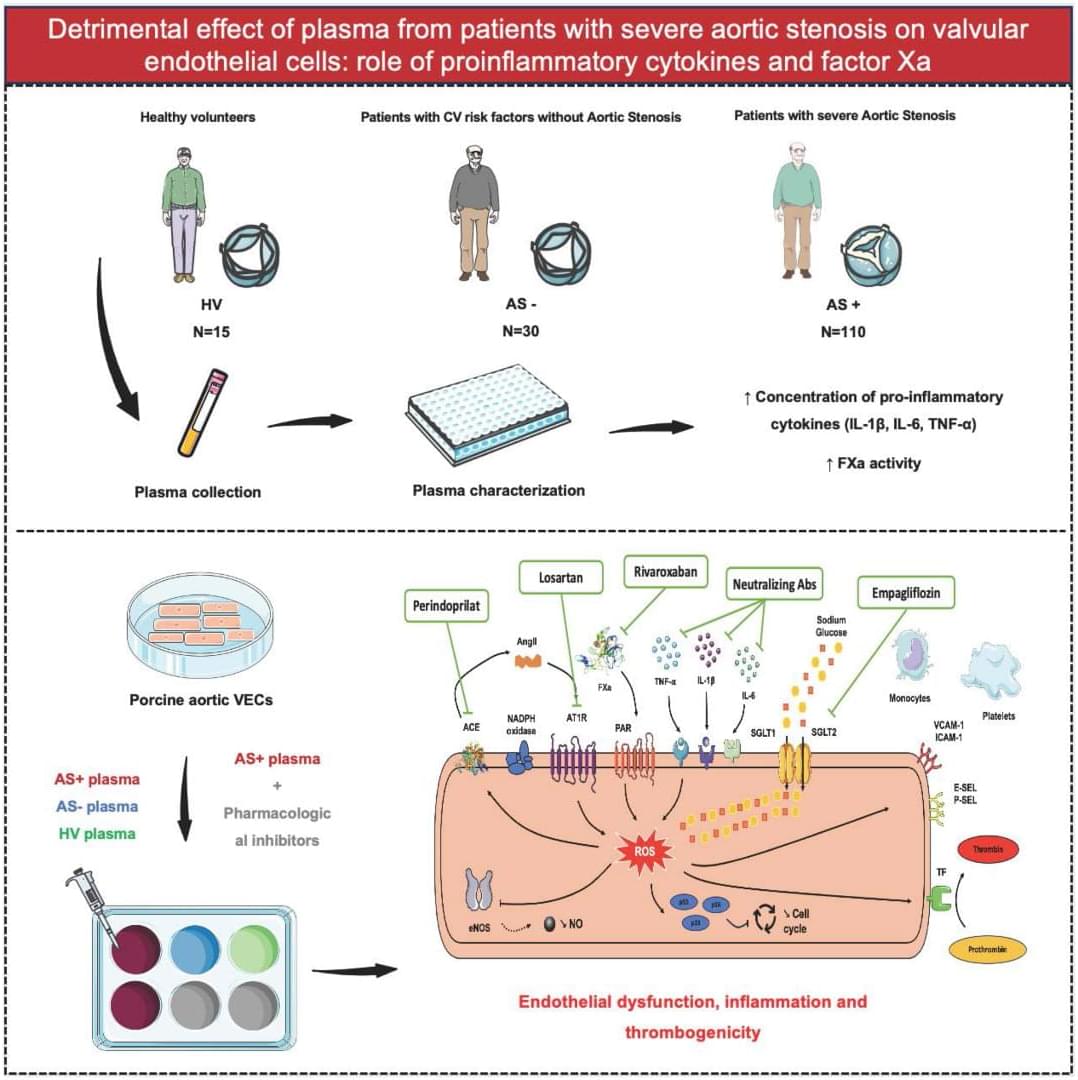
Severe AS plasma is pro-inflammatory and pro-thrombotic: it drives oxidative stress + endothelial dysfunction + monocyte/platelet adhesion in VECs. Multiple drug classes blunted these effects. @A_Trimaille @adrien_carmona @BMarchandot
Aortic stenosis (AS) is the most prevalent valvular heart disease in developed countries.1 Despite its widespread occurrence, no medical treatment has been validated to prevent the thickening of the aortic valve and the reduction in its opening area. The only available therapeutic option to date remains surgical or transcatheter aortic valve replacement once the severity criteria are met.2, 3 A comprehensive understanding of AS pathophysiology is therefore essential for identifying new therapeutic targets.
Initially thought to be a passive degenerative process, AS is now recognized as an active, multifaceted condition involving numerous cellular and molecular contributors.1, 4 The progression of AS follows a chronological sequence, beginning with the damage and dysfunction of valvular endothelial cells (VECs) due to biomechanical forces acting on the aortic valve. This damage promotes intravalvular inflammation and neoangiogenesis, followed by myofibroblastic and osteoblastic differentiation of valvular interstitial cells. Additionally, several lines of evidence suggest a bidirectional interaction between the pathomechanisms of AS and various components of the hemostatic system, including platelets, tissue factor, thrombin, von Willebrand factor, and extracellular vesicles.4, 5, 6
Given the pivotal role of VECs in maintaining valvular homeostasis under physiological conditions, and their early involvement in AS pathogenesis, they are considered key actors in the disease process. Although biomechanical factors have been identified as primary triggers of VEC dysfunction in the early stage of AS,4 the molecular mechanisms underlying the interaction between plasma from patients with AS and aortic VECs remain unclear. Because plasma contains various biological effectors potentially contributing to endothelial cell dysfunction, including proinflammatory cytokines and hemostatic factors,4, 7 this study aimed to investigate whether plasma from patients with AS induces oxidative stress and contributes to VEC dysfunction.