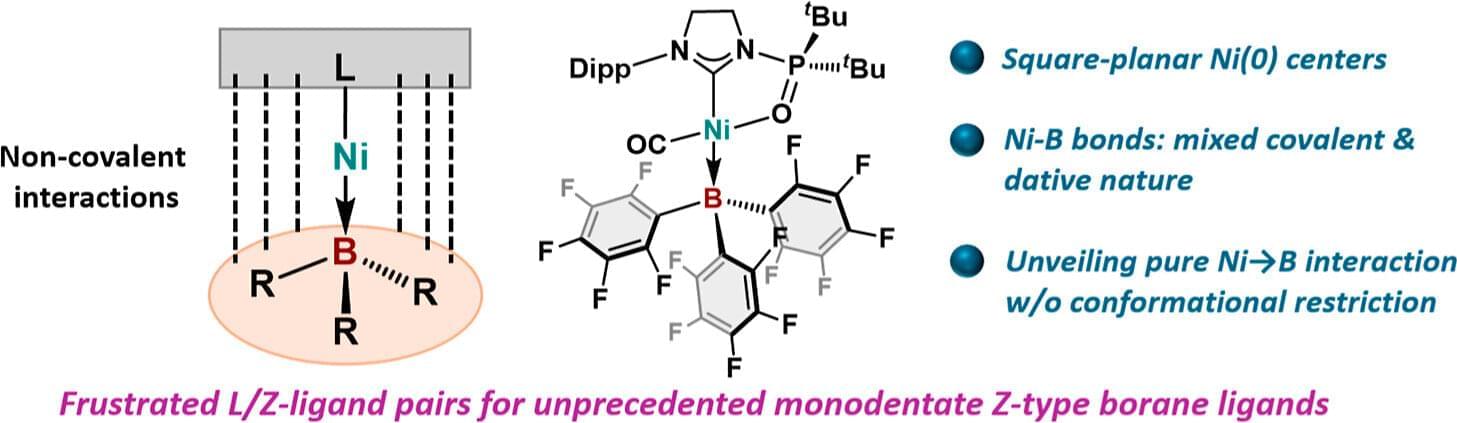
The arrangement of small molecules—known as ligands—around transition metal atoms affects how the metal atoms behave. This is important because transition metals are used as catalysts in the synthesis of a wide range of important materials.
Now, in a study published in the Journal of the American Chemical Society, researchers from the University of Osaka have reported a chemical bond that hadn’t been reported before: complexes of nickel, a metal, with simple ligands containing boron, a non-metal.
Transition metals are known to form complexes with ligands containing atoms from group 13 elements, including aluminum, gallium, and indium. These are known as Z-type ligands, and they can accept electrons from a metal. However, boron, the smallest element in group 13, has only been shown to do this with the support of additional ligands that help approach metals to the boron center.