By flying closer to the Sun than ever, Parker Solar Probe reveals the chaotic birthplace of solar wind and space storms, offering clues that may boost Earth’s defenses.
Get the latest international news and world events from around the world.

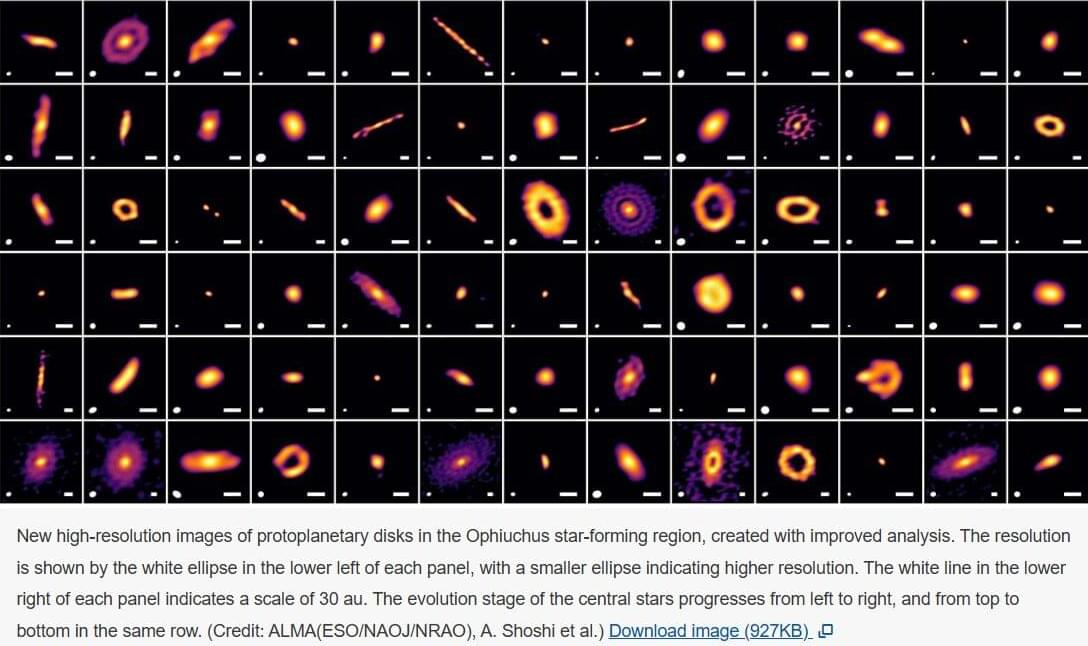
Planets may start forming before stars even finish growing
New high-resolution images of protoplanetary disks in the Ophiuchus star-forming region, created with improved analysis. The resolution is shown by the white ellipse in the lower left of each panel, with a smaller ellipse indicating higher resolution. The white line in the lower right of each panel indicates a scale of 30 au. The evolution stage of the central stars progresses from left to right, and from top to bottom in the same row. (Credit: ALMA(ESO/NAOJ/NRAO), A. Shoshi et al.)
In a stellar nursery 460 light-years away, astronomers sharpened old ALMA data and spotted crisp rings and spirals swirling around 27 infant stars—evidence that planets start taking shape just a few hundred thousand years after their suns ignite, far earlier than anyone expected.
Signs of planet formation may appear earlier than expected around still-forming baby stars, according to new results of higher resolution images produced using new improved techniques to reanalyze radio astronomy archive data. These newly discovered signs of planet formation will provide a better understanding of when it begins around a young star, thereby elucidating the process that leads to planet formation, including habitable planets like Earth.
Can we upload consciousness to computers? | Pigliucci, Yampolskiy, Sandberg, Dijkstra
Massimo Pigliucci, Roman Yampolskiy, Anders Sandberg, and Nadine Dijkstra discuss the latest developments in neuroscience and computer programming.
Can we upload our minds to computers?
With a free trial, you can watch the full debate NOW at https://iai.tv/video/consciousness-in-the-clouds?utm_source=…escription.
The idea of uploading our minds to the digital cloud has not only been taken seriously by Silicon Valley, but turned into a detailed business plan. Elon Musk claims digitising consciousness will revolutionise humanity, and the industry is estimated to be worth $50 billion by 2030. But it’s unknown whether, in principle, we can replicate minds with computer code, or whether we should seek to do so. Critics argue we have no idea how a machine could create consciousness, and neuroscientists have yet to provide an explanation for how the brain does so. A survey of specialists by Nature found the majority thought it unlikely AI would achieve consciousness anytime soon.
Should we see talk of uploading our minds to the cloud as implausible tech marketing nonsense? Should we conclude that if thought and consciousness are unobservable, it will not be possible to replicate the mind with silicon chips? Or is digital immortality such a profound and important sea change in our lives and potential that we should pursue it at all costs?
#consciousness #ai #artificialintelligence #elonmusk #neuralink #neuroscience.
Elon Just Made Tesla Unstoppable
Questions to inspire discussion.
🚀 Q: How might Elon Musk’s diverse projects contribute to Tesla’s value? A: Musk’s involvement in AI, energy, transportation, and communication through projects like Tesla, SpaceX, and Neuralink demonstrates his capacity to make progress on multiple fronts, potentially creating significant value for Tesla.
Political Involvement and Economic Strategy.
🏛️ Q: Why is Elon Musk getting involved in politics? A: Musk’s political involvement aims to create a better political system on Earth, addressing the unsustainability of US government spending and debt to avoid a fiscal doom loop.
📊 Q: What is Musk’s strategy to improve the US economy? A: Musk plans to accelerate GDP growth through AI-driven growth, humanoid bots, and reducing government spending and waste, potentially breaking free from the constant 7% growth line of the US stock market.
💰 Q: How could reducing government spending benefit the economy? A: By cutting wasteful spending and implementing a balanced budget requirement, the US could potentially grow its economy faster than its spending, reducing interest costs and freeing up money for other investments.
Rivian CEO Exposes Legacy Automaker EV Lies
Rivian CEO RJ Scaringe claims that legacy automakers are intentionally slowing down electric vehicle adoption and hindering competition to protect their profits from gas-powered vehicles, which could threaten their survival and allow newer EV makers like Rivian and Tesla to dominate the market ## ## Questions to inspire discussion.
Legacy Automakers and EVs.
🚗 Q: Why are legacy automakers resistant to selling EVs? A: Legacy automakers don’t want to sell EVs because they make good margins on low-efficiency gas cars and can sell them at a premium price, preferring to see the EV market disappear.
🏛️ Q: How are legacy automakers fighting against EV policies? A: Legacy automakers are the biggest adversaries of EV policies, spending the most energy fighting against them in DC, reflecting their desire for the EV market to vanish. Rivian’s Challenges and Strategy.
💰 Q: What financial challenge does Rivian face? A: Rivian has a massive $23 billion debt, making it more indebted than any startup has ever been, requiring 10–20 years to become cash flow positive.
🛻 Q: How is Rivian addressing its product pricing? A: Rivian’s R2 electric truck, launching in 2025, will target a **$45,000 starting price, a strategic move to make their products more accessible.
The Dawn of Tesla’s Robotaxi Network & Automated Transportation
Tesla’s launch of a robo-taxi network marks the beginning of a significant transportation disruption that will transform mobility, economy, geopolitics, and urban landscapes with the widespread adoption of electric autonomous vehicles ## ## Questions to inspire discussion.
Transportation Revolution.
🚗 Q: How will Tesla’s Robotaxi network impact transportation? A: Tesla’s Robotaxi network in Austin, Texas marks the ignition point for transportation disruption, with multiple companies competing to provide taxi rides without human drivers, potentially capturing 80–90% market share in 10–15 years.
🛢️ Q: What industries will be disrupted by autonomous electric vehicles? A: Autonomous electric vehicles will disrupt the oil and agriculture industries, as vehicles are the number one users of crude oil, and corn is the top agricultural product in the US, used to produce ethanol for gasoline.
🌆 Q: How will urban planning change with the rise of autonomous vehicles? A: Cities will repurpose parking spaces for retail, living areas, and solar panels, transforming urban planning and enabling new forms of transportation, including drones and aircraft.
Environmental Impact.