Researchers have developed a method that allows for the creation of a wider range of engineered living materials (ELMs) by side-stepping the need to always use biocompatible starting materials.
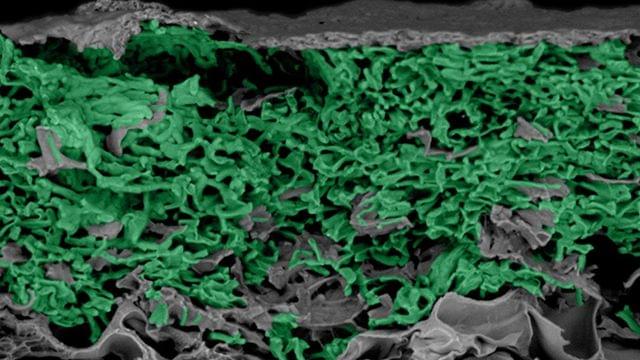


A newly developed generative AI model is helping researchers explore protein dynamics with increased speed. The deep learning system, called BioEmu, predicts the full range of conformations a protein can adopt, modeling the structural ensembles that underlie protein function.
The work, in a paper titled “Scalable emulation of protein equilibrium ensembles with generative deep learning,” was published in Science. Researchers developed BioEmu as a high-speed emulator of protein motion, capable of generating thousands of conformational states in just one GPU-hour, significantly outperforming traditional molecular dynamics (MD) simulations.
Understanding protein function has been a challenge, often hinging not on a single structural component of the protein, but on the combined ensemble of shapes within the protein. Proteins frequently shift between different conformations depending on their interactions or environment, which has been a challenge for other methods to capture accurately.
A research team led by Eske Willerslev, professor at the University of Copenhagen and the University of Cambridge, has recovered ancient DNA from 214 known human pathogens in prehistoric humans from Eurasia.
The study shows, among other things, that the earliest known evidence of zoonotic diseases—illnesses transmitted from animals to humans, like COVID in recent times—dates back to around 6,500 years ago, with such diseases becoming more widespread approximately 5,000 years ago.
“The spatiotemporal distribution of human pathogens in ancient Eurasia” is the largest study to date on the history of infectious diseases and has been published in Nature.

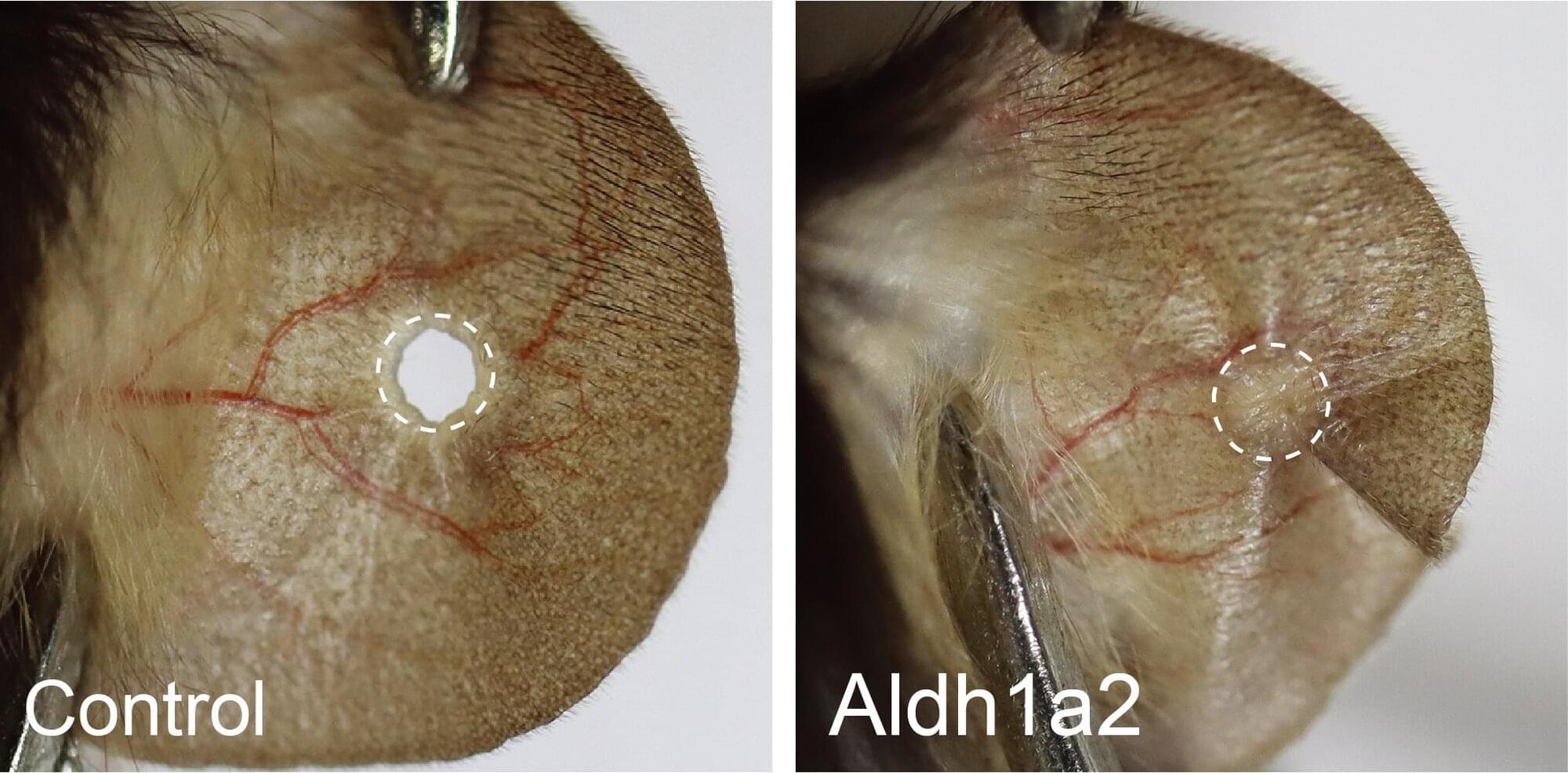
Research led by the National Institute of Biological Sciences in Beijing has discovered that switching on a single dormant gene enables mice to regenerate ear tissue.
Some vertebrates such as salamanders and fish can regenerate complex tissue structures with precision. A lost limb can be regrown, a damaged heart or eye can be repaired. Salamanders are so remarkable at reconstructing damaged tissues that even a spinal cord injury with severed neural motor connectivity can be restored.
Mammals occasionally showcase the ability to regenerate. Deer antlers and goat horns are examples of living tissue regeneration. Mice can regrow fingertips if they are lost. A healthy human liver can experience up to 70% loss of tissue and regrow to near full size within several weeks.




A new cancer drug candidate developed by Lawrence Livermore National Laboratory (LLNL), BBOT (BridgeBio Oncology Therapeutics) and the Frederick National Laboratory for Cancer Research (FNLCR) has demonstrated the ability to block tumor growth without triggering a common and debilitating side effect. In early clinical trials, the compound, known as BBO-10203, has shown promise in disrupting a key interaction between two cancer-driving proteins — RAS and PI3Kα — without causing hyperglycemia (high blood-sugar levels), which has historically hindered similar treatments. Published in Science