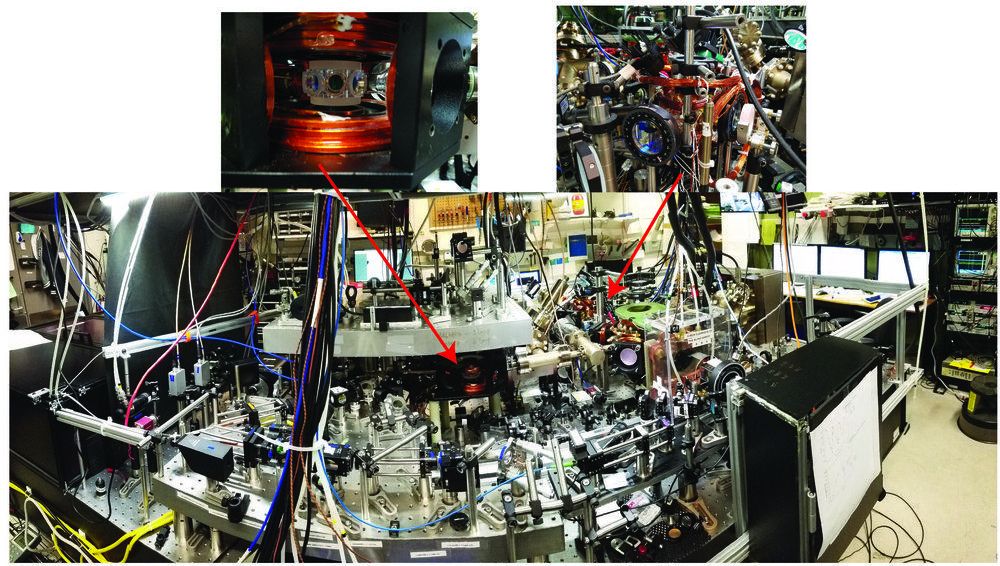
The concept of universal physics is intriguing, as it enables researchers to relate physical phenomena in a variety of systems, irrespective of their varying characteristics and complexities. Ultracold atomic systems are often perceived as ideal platforms for exploring universal physics, owing to the precise control of experimental parameters (such as the interaction strength, temperature, density, quantum states, dimensionality, and the trapping potential) that might be harder to tune in more conventional systems. In fact, ultracold atomic systems have been used to better understand a myriad of complex physical behavior, including those topics in cosmology, particle, nuclear, molecular physics, and most notably, in condensed matter physics, where the complexities of many-body quantum phenomena are more difficult to investigate using more traditional approaches.
Understanding the applicability and the robustness of universal physics is thus of great interest. Researchers at the National Institute of Standards and Technology (NIST) and the University of Colorado Boulder have carried out a study, recently featured in Physical Review Letters, aimed at testing the limits to universality in an ultracold system.
“Unlike in other physical systems, the beauty of ultracold systems is that at times we are able to scrap the importance of the periodic table and demonstrate the similar phenomenon with any chosen atomic species (be it potassium, rubidium, lithium, strontium, etc.),” Roman Chapurin, one of the researchers who carried out the study, told Phys.org. “Universal behavior is independent of the microscopic details. Understanding the limitations of universal phenomenon is of great interest.”