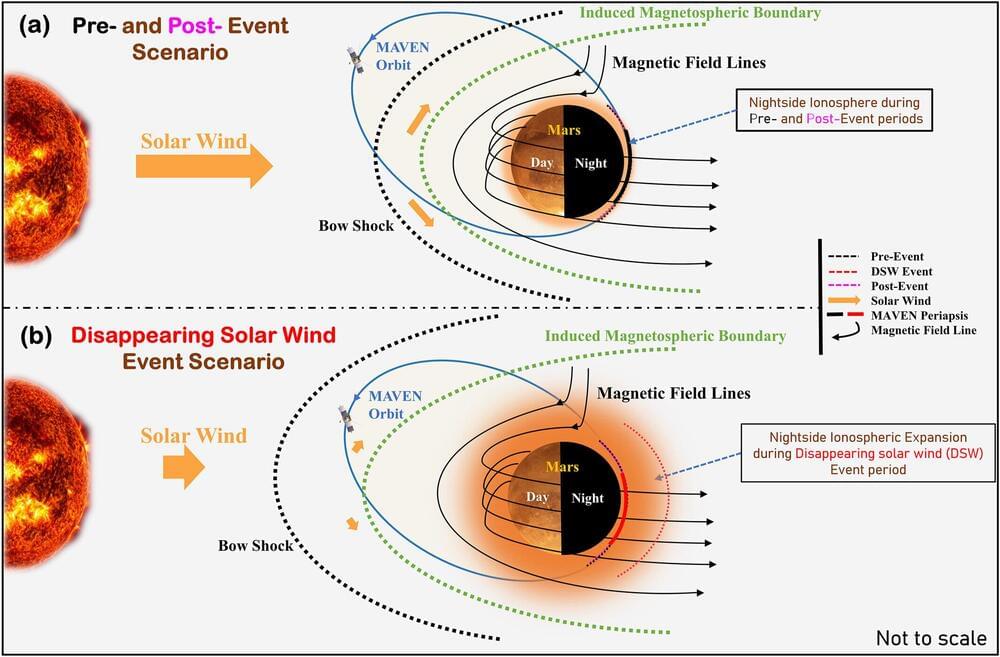
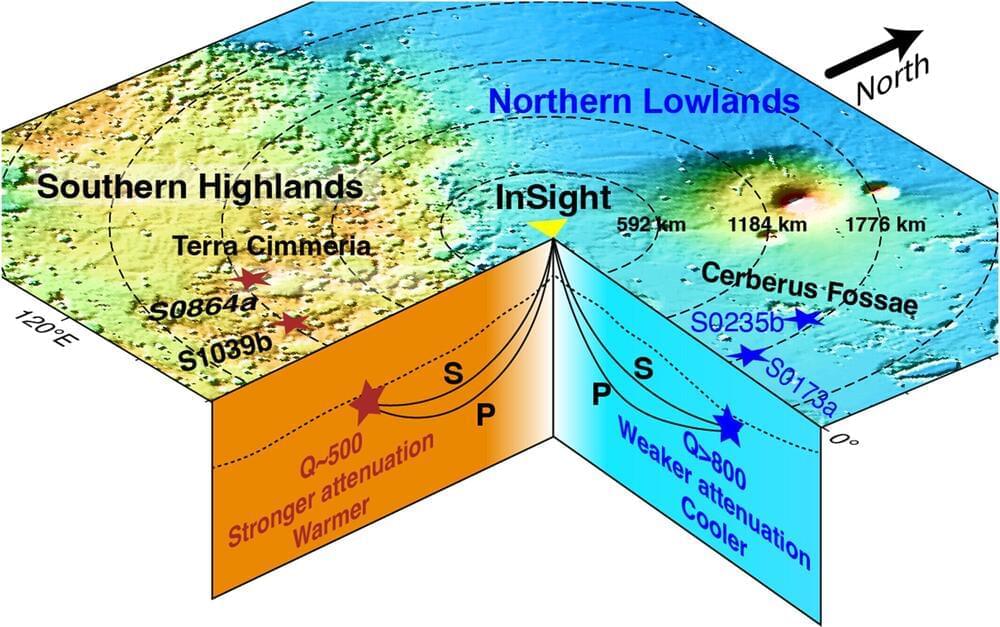
Bright, twisted light can be produced with technology similar to an Edison light bulb, researchers at the University of Michigan have shown. The finding adds nuance to fundamental physics while offering a new avenue for robotic vision systems and other applications for light that traces out a helix in space.
“It’s hard to generate enough brightness when producing twisted light with traditional ways like electron or photon luminescence,” said Jun Lu, an adjunct research investigator in chemical engineering at U-M and first author of the study on the cover of this week’s Science.
“We gradually noticed that we actually have a very old way to generate these photons—not relying on photon and electron excitations, but like the bulb Edison developed.”