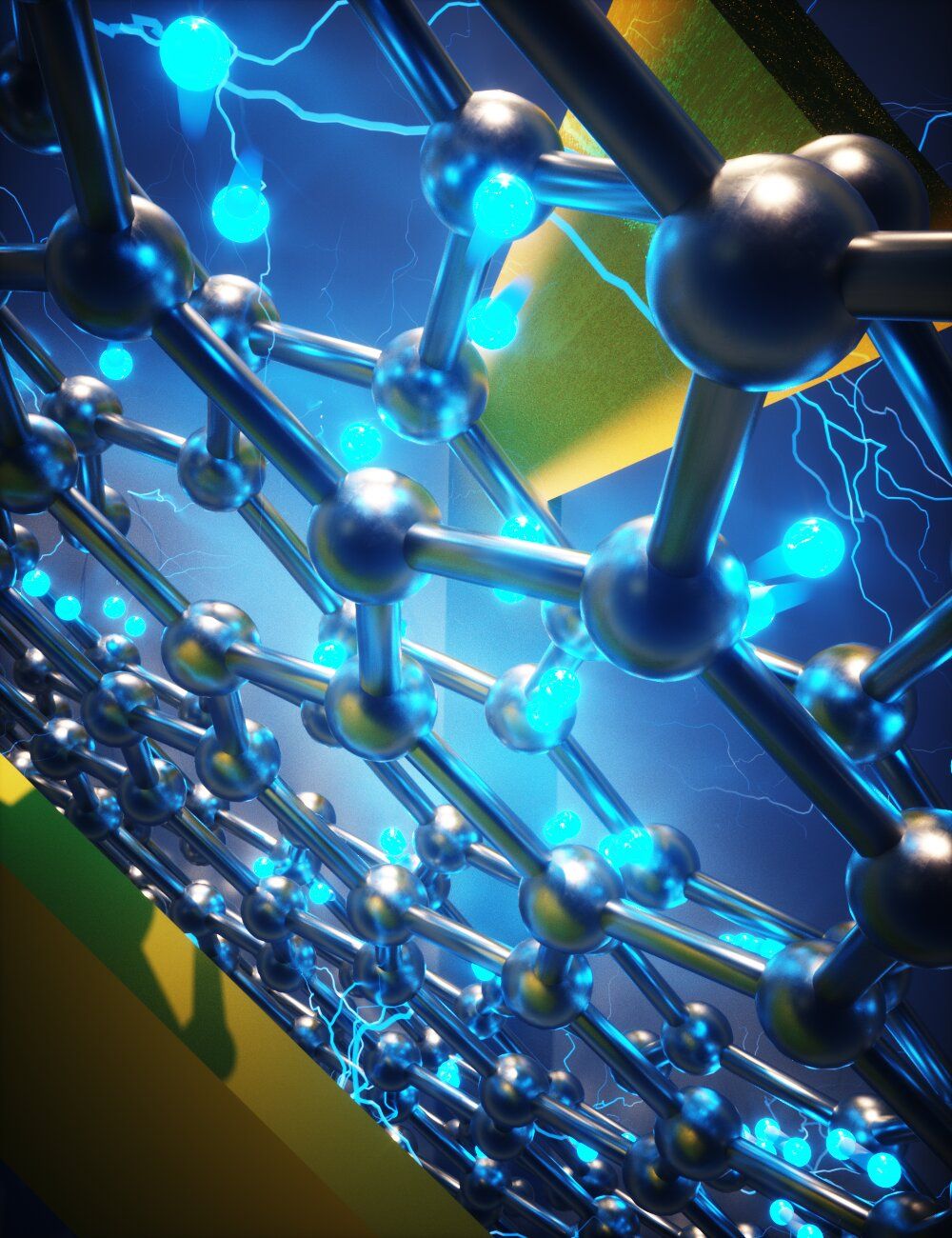
In a feat worthy of a laboratory conceived by J.K. Rowling, MIT researchers and colleagues have turned a “magic” material composed of atomically thin layers of carbon into three useful electronic devices. Normally, such devices, all key to the quantum electronics industry, are created using a variety of materials that require multiple fabrication steps. The MIT approach automatically solves a variety of problems associated with those more complicated processes.
As a result, the work could usher in a new generation of quantum electronic devices for applications including quantum computing. Further, the devices can be superconducting, or conduct electricity without resistance. They do so, however, through an unconventional mechanism that, with further study, could give new insights into the physics of superconductivity. The researchers report their results in the May 3, 2021 issue of Nature Nanotechnology.
“In this work we have demonstrated that magic angle graphene is the most versatile of all superconducting materials, allowing us to realize in a single system a multitude of quantum electronic devices. Using this advanced platform, we have been able to explore for the first time novel superconducting physics that only appears in two dimensions,” says Pablo Jarillo-Herrero, the Cecil and Ida Green Professor of Physics at MIT and leader of the work. Jarillo-Herrero is also affiliated with MIT’s Materials Research Laboratory.