From surveillance to defense to AI/ML virtualization, and it’s more compact and energy efficient. Oh and let’s not forget the medical imaging applications. I just wonder how long until it’s put into effect.
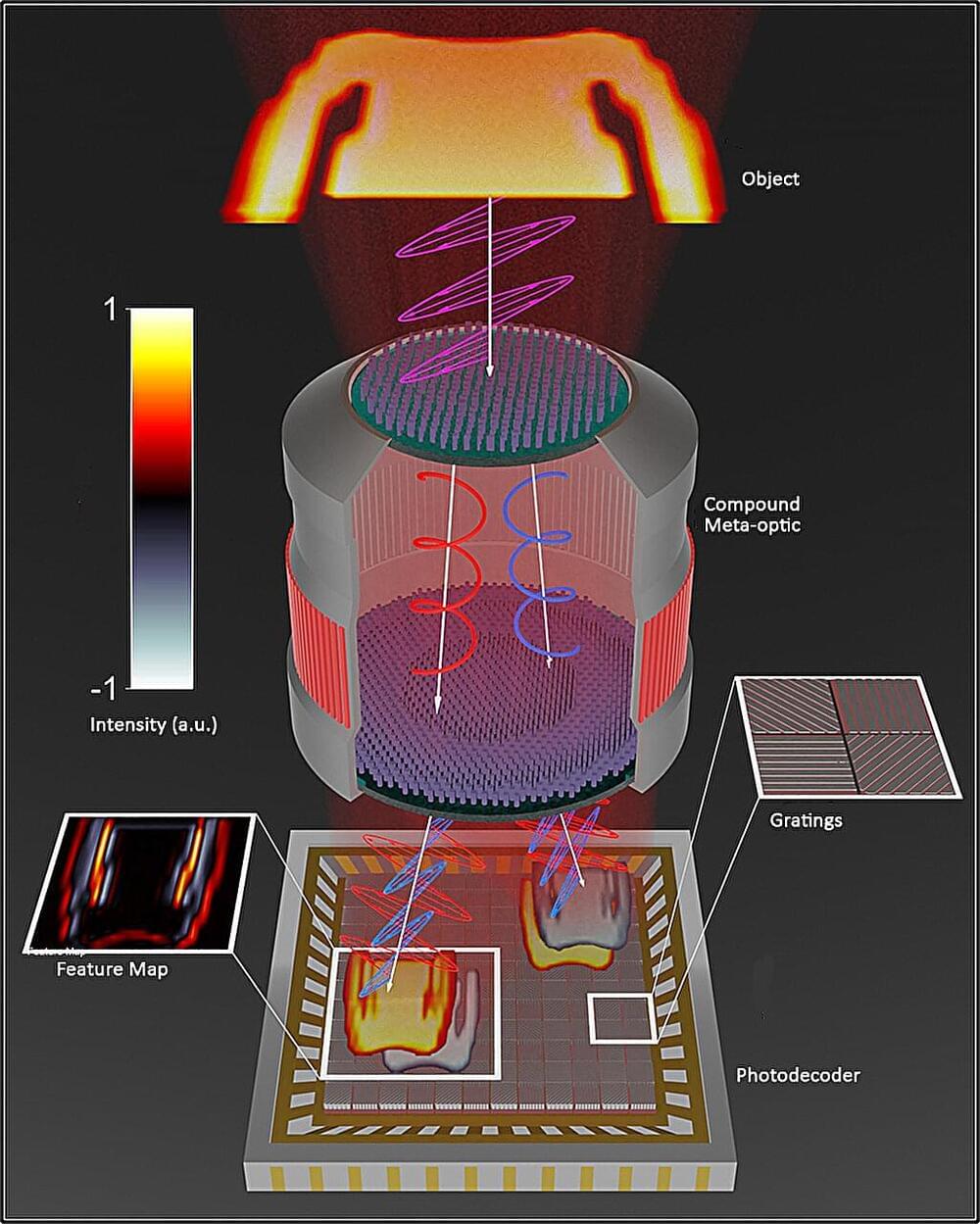
A front-end lens, or meta-imager, created at Vanderbilt University can potentially replace traditional imaging optics in machine-vision applications, producing images at higher speed and using less power.
The nanostructuring of lens material into a meta-imager filter reduces the typically thick optical lens and enables front-end processing that encodes information more efficiently. The imagers are designed to work in concert with a digital backend to offload computationally expensive operations into high-speed and low-power optics. The images that are produced have potentially wide applications in security systems, medical applications, and government and defense industries.
Mechanical engineering professor Jason Valentine, deputy director of the Vanderbilt Institute of Nanoscale Science and Engineering, and colleagues’ proof-of-concept meta-imager is described in a paper published in Nature Nanotechnology.


 עברית (Hebrew)
עברית (Hebrew)