New nanocavities pave the way for enhanced nanoscale lasers and LEDs that could enable faster data transmission using smaller, more energy-efficient devices.
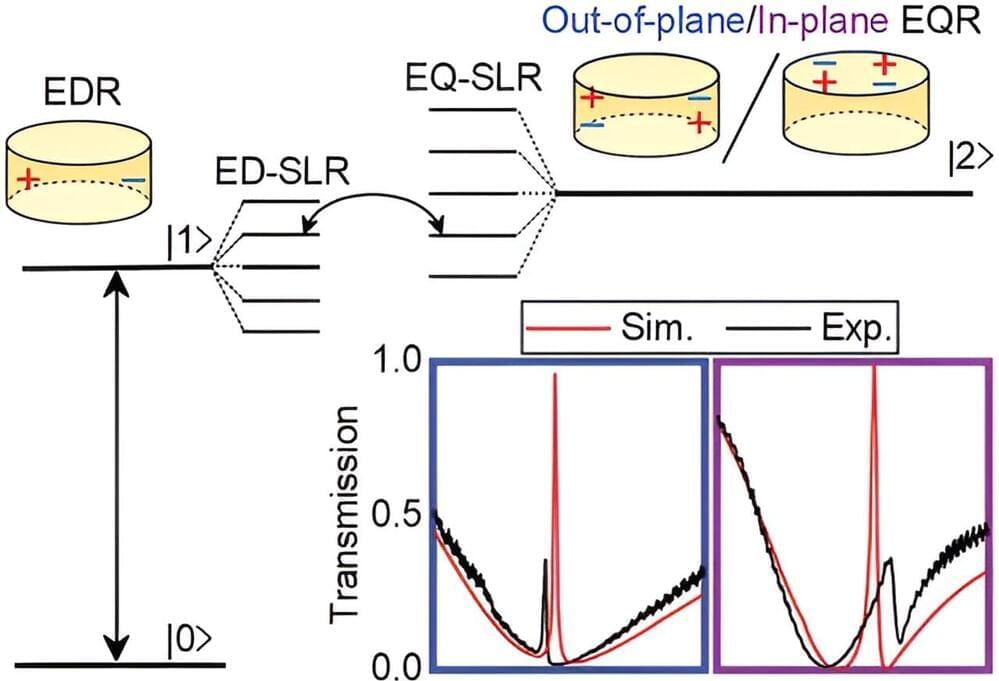
As we transition to a new era in computing, there is a need for new devices that integrate electronic and photonic functionalities at the nanoscale while enhancing the interaction between photons and electrons. In an important step toward fulfilling this need, researchers have developed a new III-V semiconductor nanocavity that confines light at levels below the so-called diffraction limit.
“Nanocavities with ultrasmall mode volumes hold great promise for improving a wide range of photonic devices and technologies, from lasers and LEDs to quantum communication and sensing, while also opening up possibilities in emerging fields such as quantum computing,” said the leading author Meng Xiong from the Technical University of Denmark. “For example, light sources based on these nanocavities could significantly improve communication by enabling faster data transmission and strongly reduced energy consumption.