The wearables market has been dominated, so far, by smartwatches and fitness trackers. The first Apple Watch was launched in April 2015, and wearable technology now includes jewelry that tracks your steps and notifies you of an incoming call, VR headsets for gamers, earbuds, smart glasses with Internet access, smart clothing integrated with electronic devices and a range of health monitors.
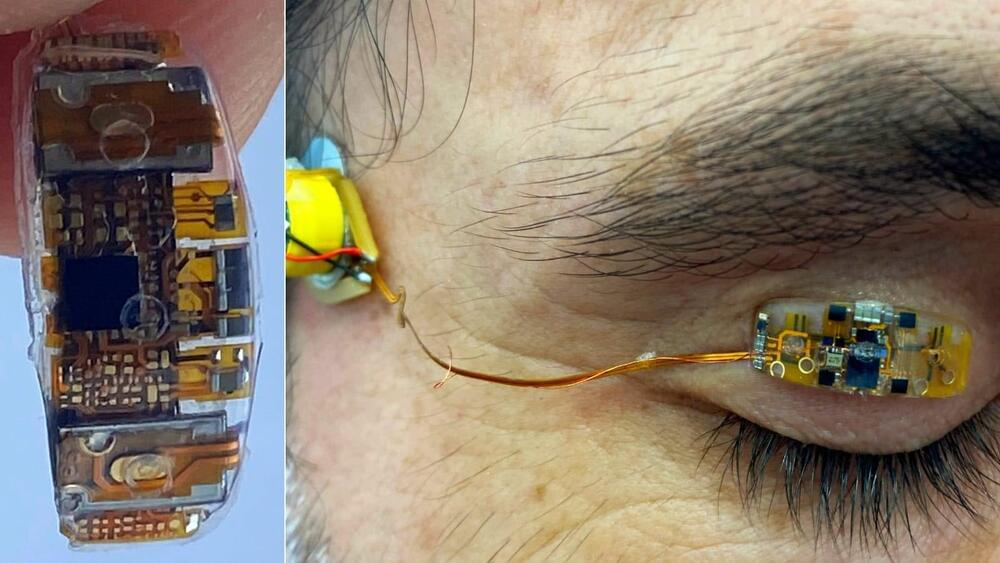
But the world’s first eyelid wearable device opens up a whole new world of opportunity.
Blink Energy’s device weighs just 0.4 grams (0.014 ounces) — less than half the weight of a paperclip – and is fitted to one eyelid. You barely notice it, says Bar-On. “After two minutes you forget it’s there.”