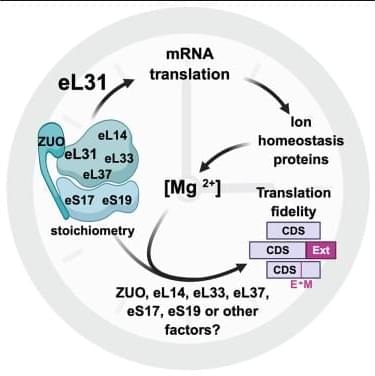
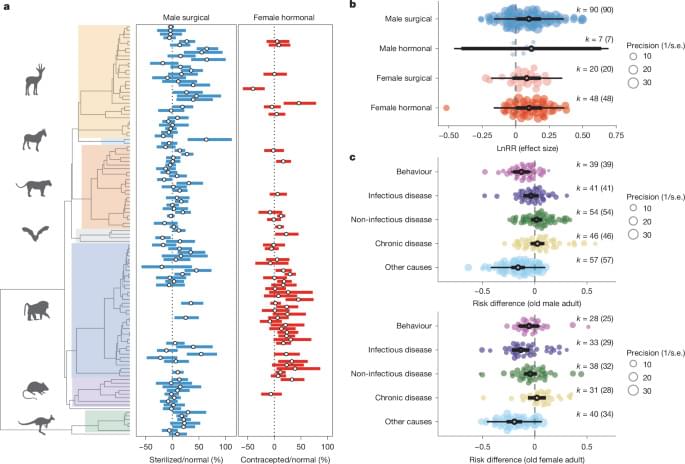
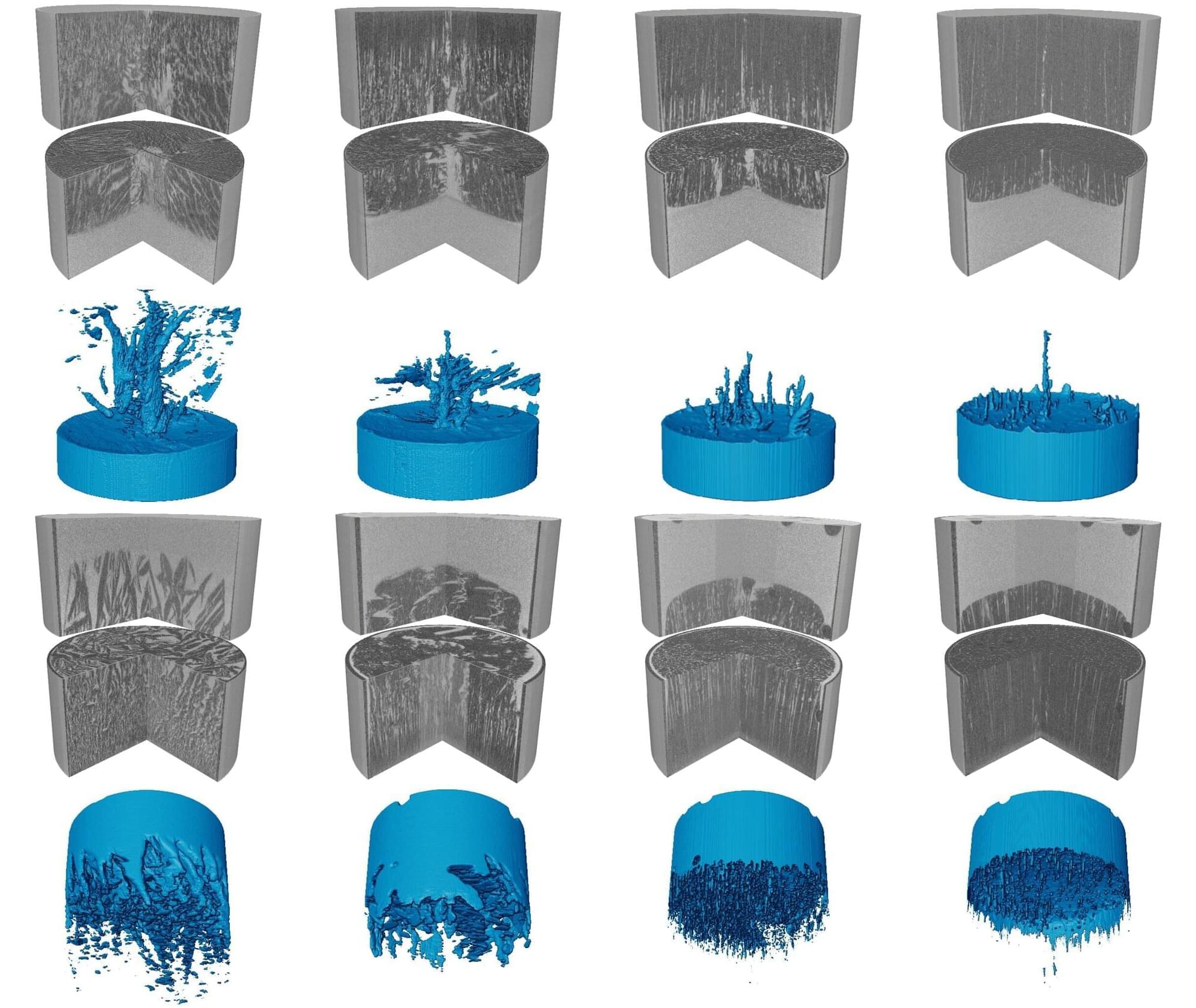
Science has a rich tradition of physics by imagination. From the 16th century, scientists and philosophers have conjured ‘demons’ that test the limits of our strongest theories of reality.
Three stand out today: Laplace’s demon, capable of perfectly predicting the future; Loschmidt’s demon, which could reverse time and violate the second law of thermodynamics; and Maxwell’s demon, which create a working heat engine at no cost.
Though imaginary, these paradoxical beings have pushed physicists towards sharper theories. From quantum theory to thermodynamics, these demons have legacies that we still feel today.
Image: Antonio Sortino
Three thought experiments involving “demons” have haunted physics for centuries. What should we make of them today?