Name: Travis Chen and Brian Femminella
Age: 22 and 21
Location: Seattle, Washington; Los Angeles, California.
Business: SoundMind, a music therapy app designed for those experiencing trauma, depression 0, and anxiety.

Name: Travis Chen and Brian Femminella
Age: 22 and 21
Location: Seattle, Washington; Los Angeles, California.
Business: SoundMind, a music therapy app designed for those experiencing trauma, depression 0, and anxiety.


And just as private space travel is now materializing, many industry observers are forecasting that the same business model will give rise to commercial fusion — desperately needed to decarbonize the energy economy — within a decade. “There’s a very good shot to get there within less than ten years,” says Michl Binderbauer, chief executive of TAE Technologies. In the FIA report, a majority of respondents thought that fusion would power an electrical grid somewhere in the world in the 2030s.
An emerging industry of nuclear-fusion firms promises to have commercial reactors ready in the next decade.

The creation of the Rolls-Royce Small Modular Reactor (SMR) business was announced following a £195m cash injection from private firms and a £210m grant from the government.
It is hoped the new company could create up to 40,000 jobs by 2050.
However, critics say the focus should be on renewable power, not new nuclear.
Private investors and the UK government will help fund the firm’s development of small nuclear reactors.
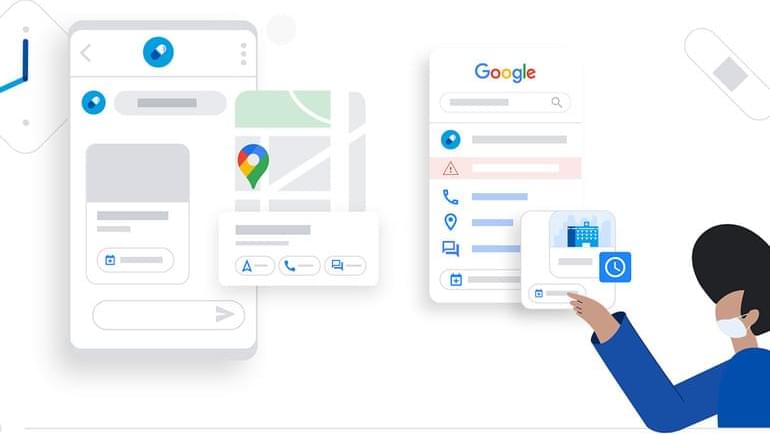
The new feature is part of Google’s Business Messages, a conversational messaging service that allows organizations to connect with people via Google Search, Google Maps, or their own business channels. For instance, Albertsons used Business Messages to share information with customers about vaccine administration. Suppose someone searched on Google for Safeway (an Albertson’s company). In that case, they could use the “message” button on Google Search to receive information like vaccine availability and how to book an appointment.
The new Bot-in-a-Box feature lets businesses launch a chatbot with an existing customer FAQ document, whether it’s from a web page or an internal document, to keep the service simple. The feature uses Google’s Dialogflow technology to create chatbots that can automatically understand and respond to customer questions without writing any code.
Qualcomm is diversifying from mobile phones, to supplying chips for BMW’s self-driving cars.
#News #Reuters #BMW #Qualcomm #SelfDriving.
Subscribe: http://smarturl.it/reuterssubscribe.
Reuters brings you the latest business, finance and breaking news video from around the globe. Our reputation for accuracy and impartiality is unparalleled.
Get the latest news on: http://reuters.com/
Follow Reuters on Facebook: https://www.facebook.com/Reuters.
Follow Reuters on Twitter: https://twitter.com/Reuters.
Follow Reuters on Instagram: https://www.instagram.com/reuters/?hl=en
The so-called metaverse has a “big time” investment case, according to Puerto Rican billionaire businessman Orlando Bravo.
Bravo, co-founder and managing partner of private equity firm Thoma Bravo, told CNBC that he thinks “metaverse” is the big word of 2021.
“It’s investable and it’s going to be very big,” Bravo said in an interview with CNBC’s Annette Weisbach on Friday.
In explosive development, China has been threatening U.S. executives, companies and business groups in recent weeks to fight against China-related bills in the U.S. Congress. According to Reuters, letters from China’s embassy in Washington have pressed executives to urge members of Congress to alter or drop specific bills that seek to enhance U.S. competitiveness.
Follow our Telegram Channel — https://t.me/tfiglobal.
Please support us on Patreon — https://www.patreon.com/tfiglobal

Many companies are experimenting with AI, often in many different areas of the company. But experimentation is not enough. It’s essential to have a bold corporate strategy driven from the top. The AI strategy needs to be coordinated throughout the company in tight alignment with the overall business strategy.
AI can be scary for employees, and many are worried about their jobs (see another jobs?). The most successful AI-fueled organizations lead with change management to enable AI systems to succeed.
A key factor for successful change management is trust. Companies that focus on relationship-building, collaboration, and training engender trust. Successful companies help employees understand that most AI replaces tasks, not jobs. Those companies are aiming to augment jobs so that AI and employees work together. If AI is going to replace jobs, management needs to be clear about exactly how that will play out for the employees and provide opportunities for them to upskill or reskill.
Full Story:
Deloitte recently published a comprehensive study of the current state of AI, asking the question: “What are today’s most AI-fueled organizations doing differently to drive success?”
In this article, I provide you with a summary of their findings to determine whether or not you need to go deeper to get more value.
We explore human enhancement and personal performance hacking with Matt Ward (@mattwardio), host of The Disruptors podcast, startup investor, adviser, and business innovation consultant. Matt and I thought it would be fun to do two episodes, one here on MIND & MACHINE and the other on The Disruptors, where we explore what we’ve learned, the ideas we’ve formed and our takeaways across all these different fields that we cover.
So with this episode here on MIND & MACHINE, we focus on human enhancement — technologies that are extending lifespan and enhancing human capability. Then we get into what Matt and I are doing currently to maximize our own performance capabilities — our ability to think more clearly, to live more energetic vibrant lives… which is all heavily informed by all these amazing guests across these different fields that we explore.
In the other part of this discussion, on The Disruptors, we look at another set of subjects from space to AI to Augmented and Virtual reality. So I encourage you to check that out as well at The Disruptors… For the other part of the Conversation on The Disruptors: https://is.gd/mv1Vez https://youtu.be/PtpwgTr4GSU __________ MIND & MACHINE features interviews by August Bradley with bold thinkers and leaders in transformational technologies. Subscribe to the MIND & MACHINE newsletter: https://www.mindandmachine.io/newsletter MIND & MACHINE Website: https://www.MindAndMachine.io Subscribe to the podcast on: iTunes: https://www.mindandmachine.io/itunes Android or Other Apps: https://www.mindandmachine.io/android Show Host August Bradley on Twitter: https://twitter.com/augustbradley _____________________________
For the other part of the Conversation on The Disruptors:
https://is.gd/mv1Vez.
__________
MIND & MACHINE features interviews by August Bradley with bold thinkers and leaders in transformational technologies.