2024 This is a first of its kind univversal covid 19 vaccine that would allow for a one time shot that is good for current versions of the virus and future versions aswell.
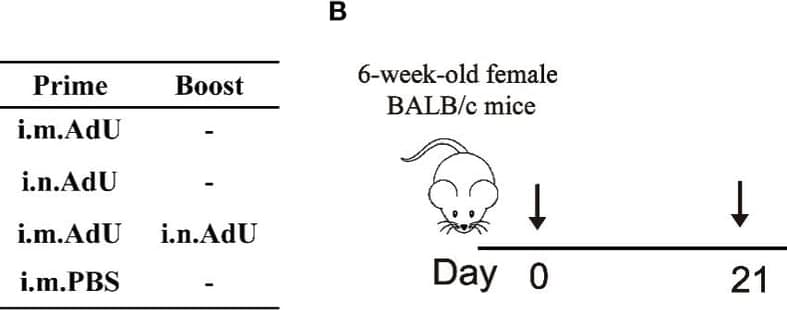
A universal recombinant adenovirus type-5 (Ad5) vaccine against COVID19 (Ad-US) was constructed, and immunogenicity and broad-spectrum of Ad5-US were evaluated with both intranasal and intramuscular immunization routes. The humoral immune response of Ad5-US in serum and bronchoalveolar lavage fluid were evaluated by the enzyme-linked immunosorbent assay (ELISA), recombinant vesicular stomatitis virus based pseudovirus neutralization assay, and angiotensin-converting enzyme-2 (ACE2)-binding inhibition assay. The cellular immune response and Th1/Th2 biased immune response of Ad5-US were evaluated by the IFN-γ ELISpot assay, intracellular cytokine staining, and Meso Scale Discovery (MSD) profiling of Th1/Th2 cytokines. Intramuscular priming followed by an intranasal booster with Ad5-US elicited the broad-spectrum and high levels of IgG, IgA, pseudovirus neutralizing antibody (PNAb), and Th1-skewing of the T-cell response. Overall, the adenovirus type-5 vectored universal SARS-CoV-2 vaccine Ad5-US was successfully constructed, and Ad5-US was highly immunogenic and broad spectrum. Intramuscular priming followed by an intranasal booster with Ad5-US induced the high and broad spectrum systemic immune responses and local mucosal immune responses.
According to the World Health Organization, as of March 15, 2024, over 774 million COVID-19 cases and almost 7 million related deaths had been reported globally. A total of 13.59 billion doses of COVID-19 vaccines have been reportedly administered globally, leading to a vaccination rate of 69.7%. At present, at least 24 types of COVID-19 vaccines have been authorized for emergency use in various countries. According to the list of global COVID-19 candidate vaccines published on the WHO website, a total of 183 candidate vaccines are in clinical development and 199 candidate vaccines are in the preclinical evaluation stage as of April 8, 2023. Recombinant protein subunit vaccines account for the greatest proportion of vaccines in clinical development (59 vaccines, 32%), followed by RNA vaccines (43 vaccines, 24%).