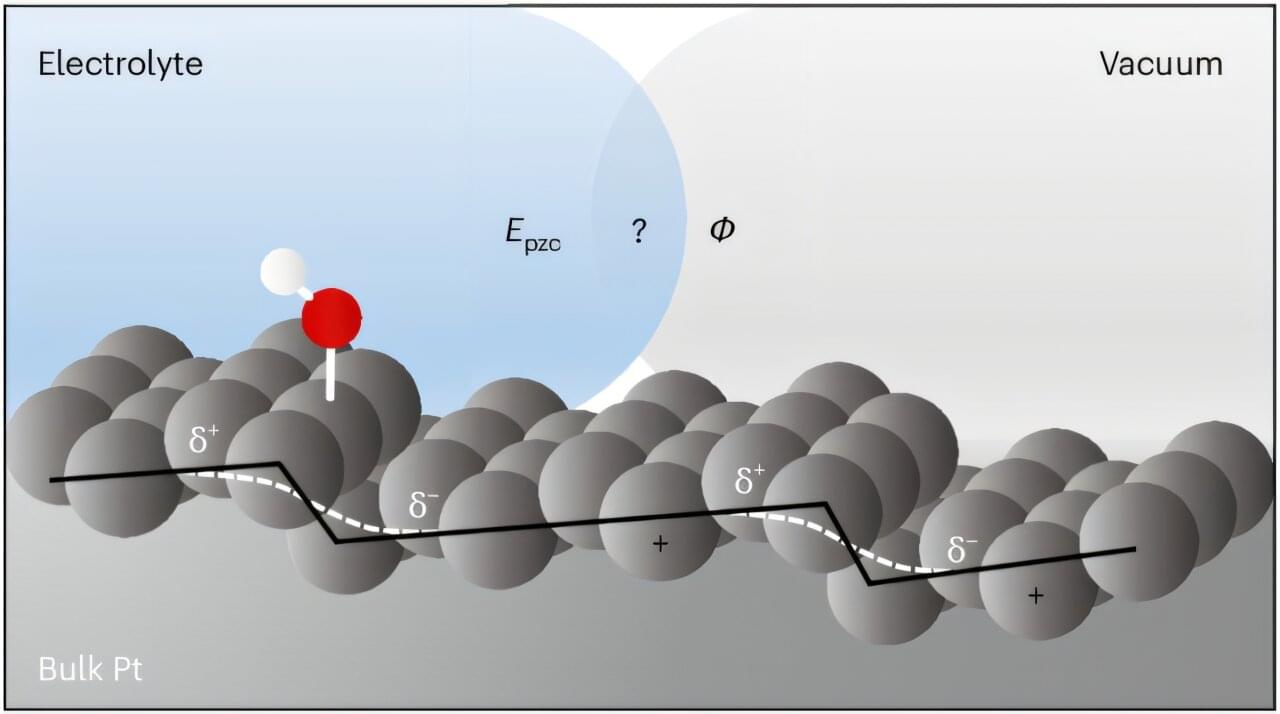
Current electrochemical theory does not adequately describe realistic platinum electrodes. Scientists at Leiden University have now, for the first time, mapped the influence of imperfect platinum surfaces. This provides a more accurate picture of these electrodes, with applications in hydrogen production and sensors.
Platinum electrodes play a crucial role in electrochemical applications. They are used in sensors, catalysis and fuel cells, for example in the production of green hydrogen. These developments call for a better and more realistic understanding of the underlying fundamental electrochemistry. Current theory falls short.
The surface of a platinum electrode appears smooth. But if you zoom in to the atomic level, you see an irregular landscape with so-called defects. These turn out to influence the electrochemical reactions that take place there. Ph.D. candidates Nicci Lauren Fröhlich and Jinwen Liu investigated this influence under the supervision of Professor Marc Koper and Assistant Professor Katharina Doblhoff-Dier at the Leiden Institute of Chemistry. Their results are published in Nature Chemistry.