As for decay accelerating factor (DAF); also known as CD55, it is a type I cell surface protein that forms a single chain anchored to the membrane by glycosylphosphatidylinositol (GPI). It binds C3b and C4b inhibiting thereby the formation of C3 convertase and decreasing its half-life, thus providing a protective barrier threshold for plasma membranes of normal autologous cells against complement deposition and activation9,10.
The role of the complement system in cancer is complicated and has been debated for long. Malignant transformation is generally accompanied by genetic and epigenetic modifications which drastically alter patterns of glycosylation, cell-surface proteins and phospholipids11. These alterations can be identified by innate and adaptive immune mechanisms that guard the host against cancer development12. This is the known basis of the immune surveillance hypothesis. There is no direct evidence to support the argument that complement is able to eradicate emerging tumors. Nevertheless, taking into consideration that complement is intended for the recognition of non-self-elements, it is assumed that alterations in the tumor cell membranes’ composition render these cells as targets for complement recognition13. However, the relationship between inflammation and cancer is complicated and subject to contradictory forces14. Therefore, while acute responses are considered a vital part of the defense against cancerous cells, continuous inflammation in the tumor microenvironment increases the threat of neoplastic transformation and has several tumor-promoting effects15.
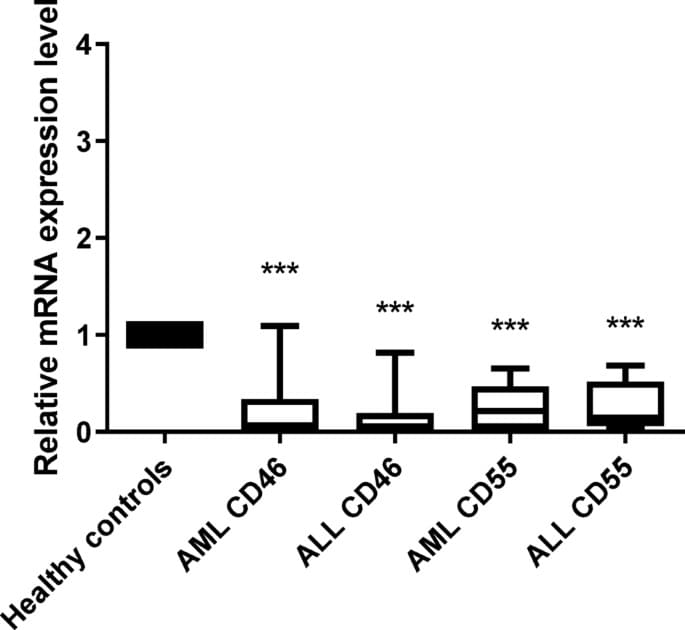
The current study aims at investigating the expression levels of mCRPs; CD46 and CD55 in the acute lymphocytic leukemia and acute myelogenous leukemia and to further elucidate its role in Egyptian cancer patients. To the best of our knowledge this study is one of very few studies tackling the complicated role of the complement system in acute leukemia.