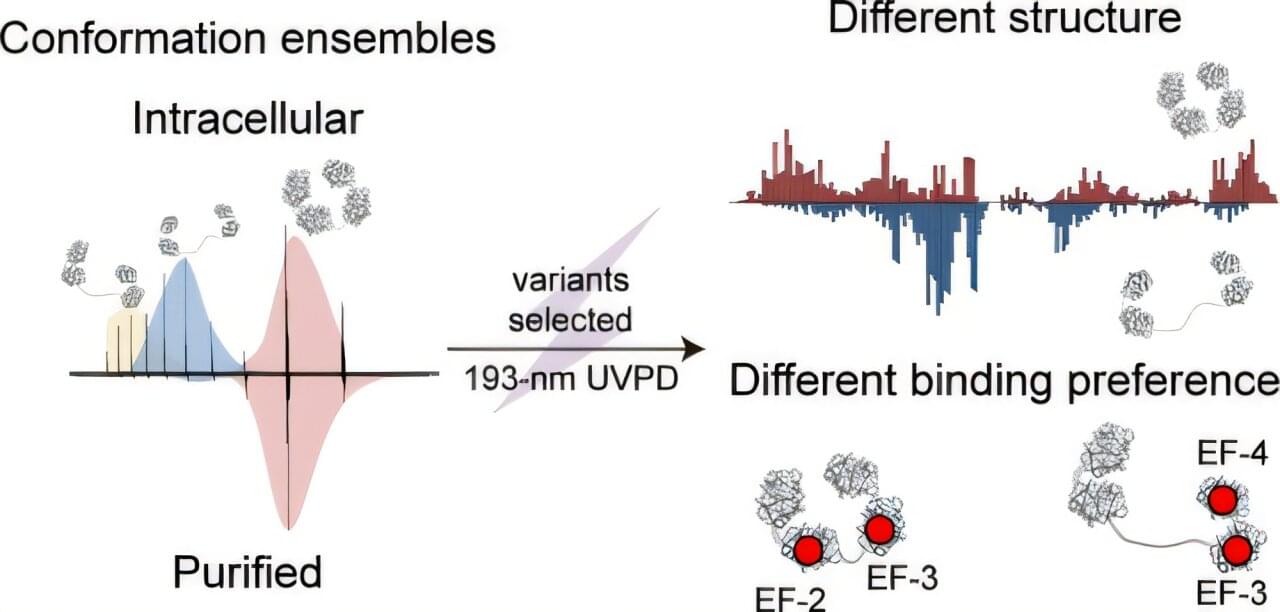
Proteins in cells are highly flexible and often exist in multiple conformations, each with unique abilities to bind ligands. These conformations are regulated by the organism to control protein function. Currently, most studies on protein structure and activity are conducted using purified proteins in vitro, which cannot fully replicate the complexity of the intracellular environment and may be influenced by the purification process or buffer conditions.
In a study published in the Journal of the American Chemical Society, a team led by Prof. Wang Fangjun from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences (CAS), collaborating with Prof. Huang Guangming from the University of Science and Technology of China of CAS, developed a new method for in-cell characterization of proteins using vacuum ultraviolet photodissociation top-down mass spectrometry (UVPD-TDMS), providing an innovative technology for analyzing the heterogeneity of intracellular protein in situ with MS.
Researchers combined in-cell MS with 193-nm UVPD to directly analyze protein structures within cells. This method employed induced electrospray ionization, which ionizes intracellular proteins with minimal structural perturbation.