Breast cancer is the most diagnosed cancer among women globally1. In the past decade, multimodal approaches and innovative therapies have transformed the outlook of this lethal disease, leading to gains in patient survival2. Despite these advances, nearly 685,000 women die of breast cancer each year worldwide1, largely due to the development of incurable distant metastases to vital organs3. In this context, a potentially critical factor may lie within the underlying principles of most anticancer drugs. Standard-of-care treatments are typically developed on the basis of their cytotoxic activity and are not necessarily designed to interfere with metastasis-relevant mechanisms4,5. Consequently, there is an intriguing yet uncharted opportunity for the development of metastasis-targeted agents that disrupt the causes of metastasis themselves4,5.
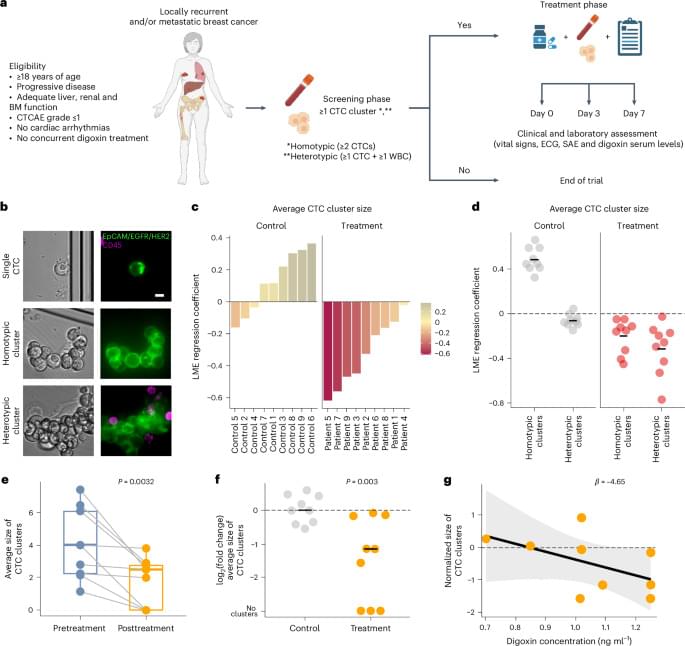
Circulating tumor cells (CTCs) are living cells that are shed from both primary and metastatic lesions into the bloodstream, acting as metastatic pioneers6. The presence of CTCs has been firmly established to be predictive of poor prognosis in patients with breast cancer7. Recent studies by us and others demonstrated that clusters of CTCs, defined as multicellular aggregates of cancer cells alone (homotypic) or in liaison with immune cells (heterotypic), have a substantially higher metastatic capacity and a stronger association with a poor prognosis than single CTCs8,9,10. Preclinical studies further revealed unique biological properties and vulnerabilities of these clusters, such as stem-like and proliferation features dependent upon cell–cell adhesion integrity8,11. A screen with 2,486 US Food and Drug Administration-approved drugs demonstrated that Na+/K+ ATPase inhibitors, such as cardiac glycosides, effectively dissolve CTC clusters into single cells, leading to metastasis suppression in mouse models of breast cancer11. Consequently, the Digoxin Induced Dissolution of CTC Clusters (DICCT) trial has been set up as a multicentric, prospective, first-in-human proof-of-concept, single-arm, therapeutic exploratory phase 1 study aimed to examine whether the Na+/K+ ATPase inhibitor digoxin could disrupt CTC clusters in patients with metastatic breast cancer at dose levels that are safe and well tolerated (NCT03928210; DICCT/Swiss-GO-07).
The primary objective of the study was to assess the effect of digoxin on CTC cluster size in patients with metastatic breast cancer. Of note, the size of CTC clusters, rather than their general abundance, best reflects cluster-dissolution properties. Secondary objectives included the effect of digoxin on the overall abundance of CTC clusters, the kinetics of CTC cluster dissolution and the dose–response relationship of the effect. Patients aged 18 years or older with locoregionally recurrent or metastatic breast cancer with progressive disease not amenable to treatments with curative intent were eligible for study inclusion. A total of 58 patients were screened by means of peripheral blood sampling and CTC cluster assessment. Of these, 11 patients resulted positive for CTC clusters at baseline, were enrolled in DICCT and received digoxin at 0.125–0.250 mg per day (intention-to-treat population) (Fig. 1a).