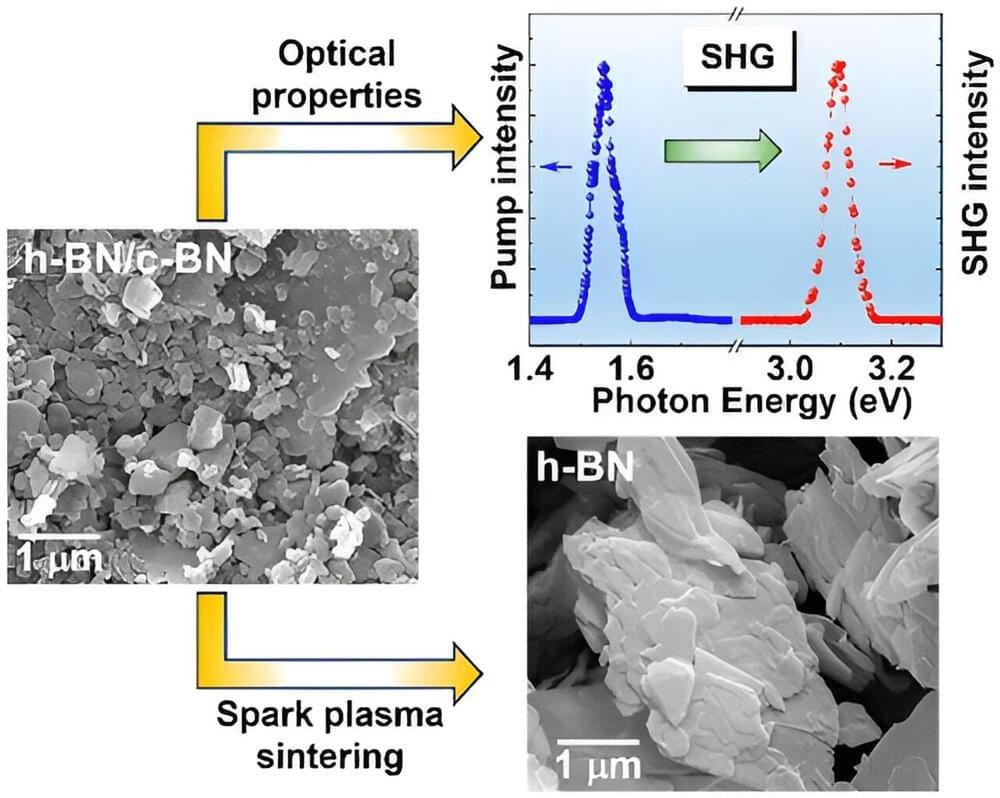
In chemistry, structure is everything. Compounds with the same chemical formula can have different properties depending on the arrangement of the molecules they’re made of. And compounds with a different chemical formula but a similar molecular arrangement can have similar properties.
Graphene and a form of boron nitride called hexagonal boron nitride fall into the latter group. Graphene is made up of carbon atoms. Boron nitride, BN, is composed of boron and nitrogen atoms. While their chemical formulas differ, they have a similar structure —so similar that many chemists call hexagonal boron nitride “white graphene.”
Carbon-based graphene has lots of useful properties. It’s thin but strong, and it conducts heat and electricity very well, making it ideal for use in electronics.