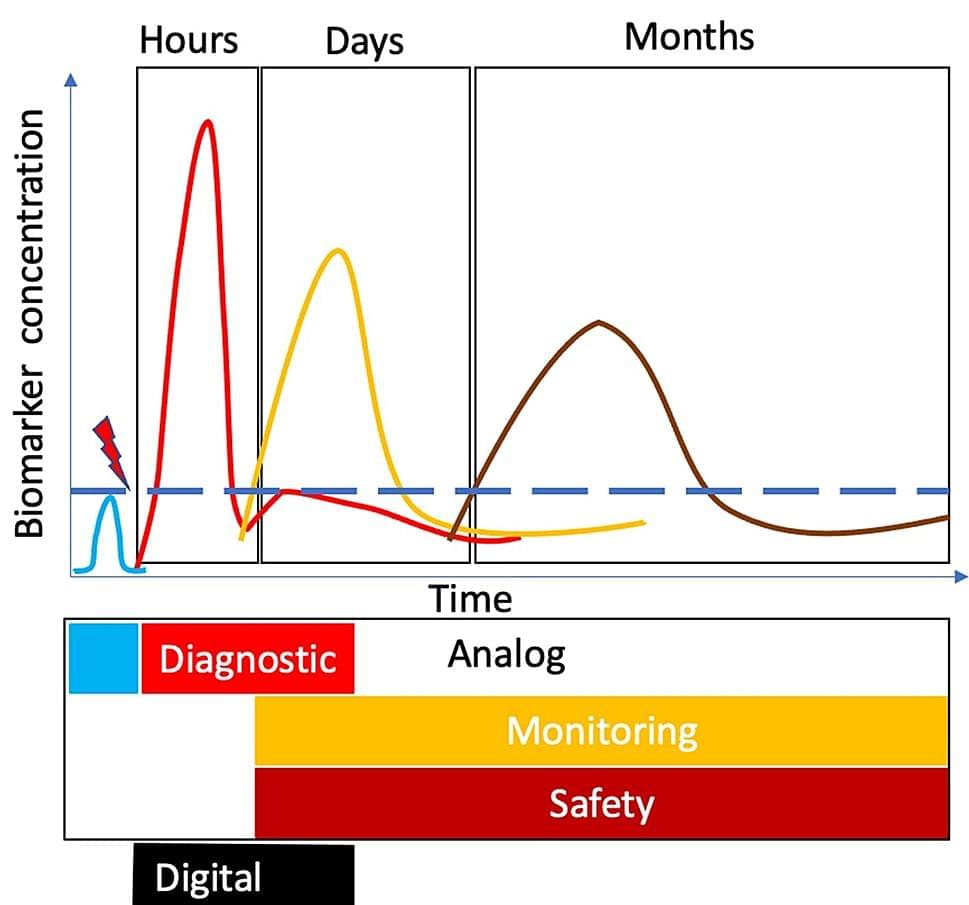
Traumatic brain injury (TBI) is increasingly a major cause of disability across the globe. The current methods of diagnosis are inadequate at classifying patients and prognosis. TBI is a diagnostic and therapeutic challenge. There is no Food and Drug Administration (FDA)-approved treatment for TBI yet. It took about 16 years of preclinical research to develop accurate and objective diagnostic measures for TBI. Two brain-specific protein biomarkers, namely, ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein, have been extensively characterized. Recently, the two biomarkers were approved by the FDA as the first blood-based biomarker, Brain Trauma Indicator™ (BTI™), via the Breakthrough Devices Program. This scoping review presents (i) TBI diagnosis challenges, (ii) the process behind the FDA approval of biomarkers, and (iii) known unknowns in TBI biomarker biology.