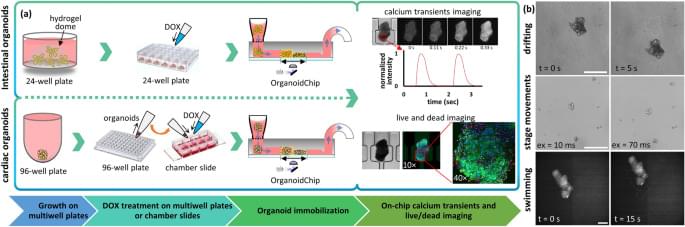
To show the capability of the OrganoidChip in enabling higher-resolution imaging, we used confocal microscopy for several organoids immobilized on the chip. Representative images show improved optical segmentation and the ability to resolve single cells within an organoid (Fig. 4 d). The co-localized EthD-1-and Hoechst-stained nuclei are resolvable and can potentially be used to increase the accuracy of viability measurements. Future implementation of 3D-segmentation using AI-assisted algorithms in the analysis pipeline can provide more accurate estimations of cellular viability in larger screens.
Next, we measured the effect of DOX treatment on the beating kinetics of cardiac organoids. To do this, we relied on calcium fluorescence imaging, as it has been shown to be a good approximation of the cardiomyocytes’ action potentials32. Calcium imaging proved beneficial for beating and contraction parameters since smaller beating portions cannot necessarily be detected from brightfield images, particularly when organoids have been compromised as a result of drug treatment.
When assessing drug effects, we observed some degree of variability in the spontaneous contractile behaviour and beating kinetics between cardiac organoids. Such variability often skews any averaged parameter value across organoids and does not reflect the effect of the treatment conditions on organoid health. To address this challenge, we tracked each individual organoid’s beating off-and on-chip. The drug-induced functionality results are therefore reported as averages of fractional changes of each individual organoid’s beating kinetics parameters, measured at 48 h post-treatment, on both the chamber slide and on the chip, relative to its pre-treatment value (Eq. 3).