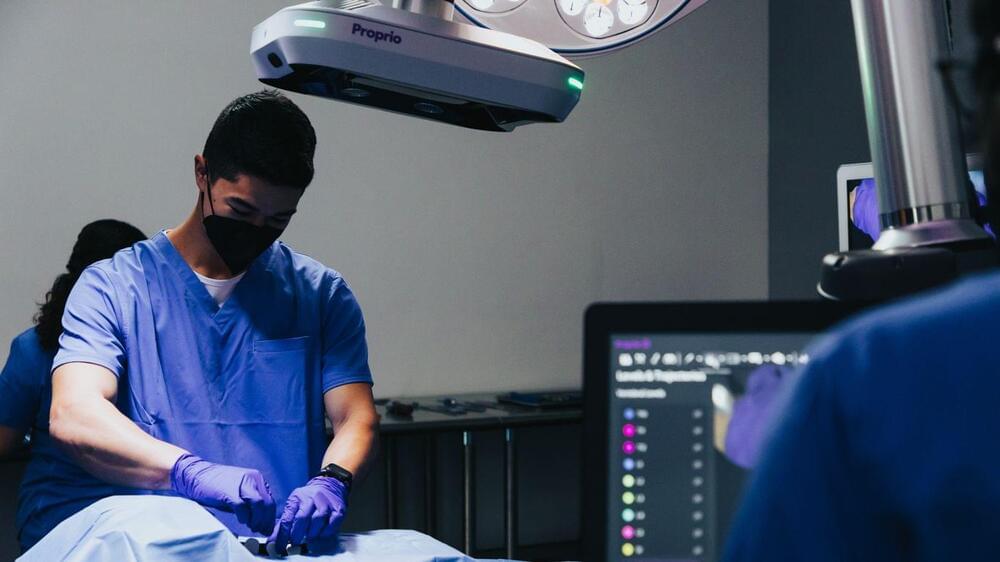
A Seattle medical tech company’s revolutionary surgical imaging instrument has become the first light-enabled navigation tool for spine surgery to receive Food and Drug Administration clearance.
Why it matters: Officials with Proprio believe the tool, which allows surgeons to essentially “see” the spine being operated on in real time, could dramatically improve clinical outcomes.
Driving the news: This month, the Paradigm device received the FDA 510(k) clearance that’s required for new medical implements before they are taken to market, Proprio said in an emailed announcement.