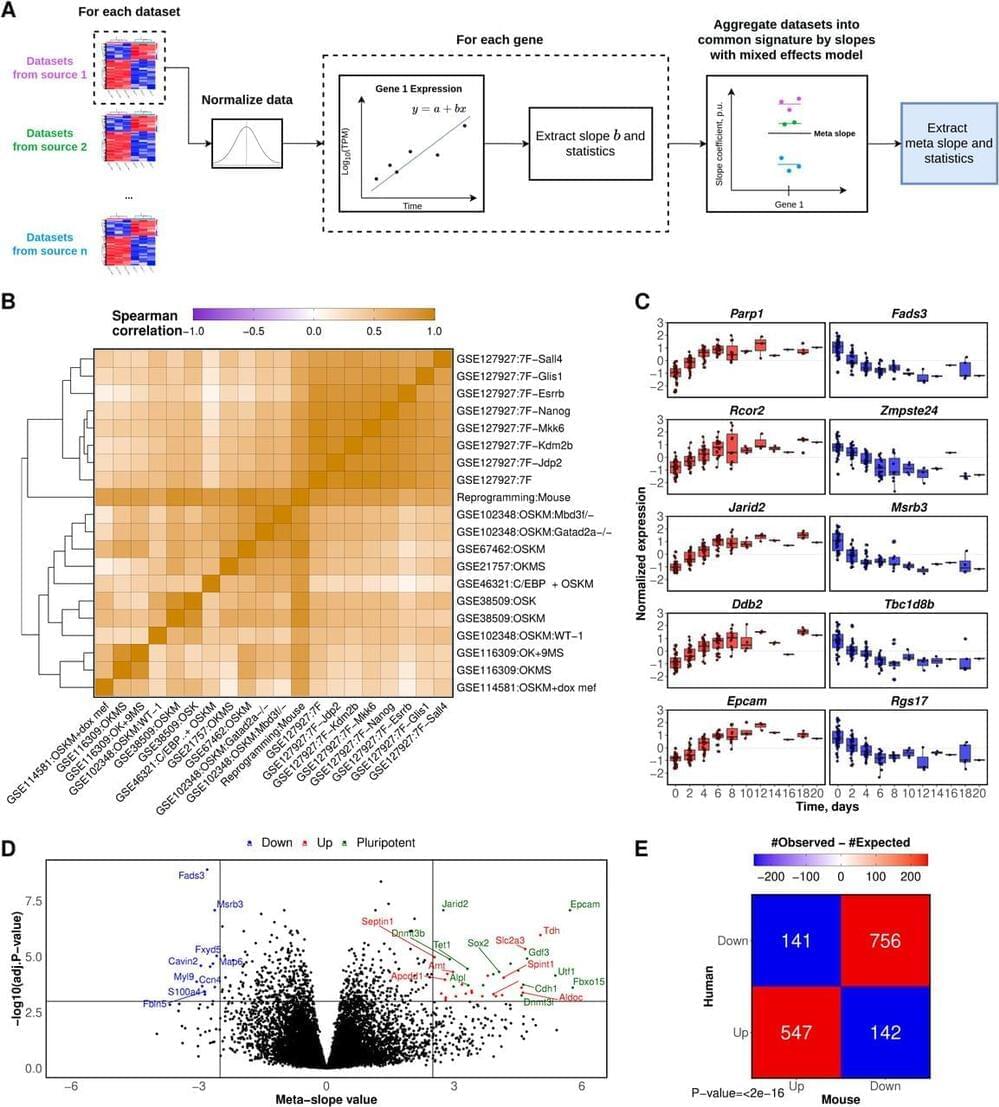
Partial somatic cell reprogramming has been touted as a promising rejuvenation strategy. However, its association with mechanisms of aging and longevity at the molecular level remains unclear. We identified a robust transcriptomic signature of reprogramming in mouse and human cells that revealed co-regulation of genes associated with reprogramming and response to lifespan-extending interventions, including those related to DNA repair and inflammation. We found that age-related gene expression changes were reversed during reprogramming, as confirmed by transcriptomic aging clocks. The longevity and rejuvenation effects induced by reprogramming in the transcriptome were mainly independent of pluripotency gain. Decoupling of these processes allowed predicting interventions mimicking reprogramming-induced rejuvenation (RIR) without affecting somatic cell identity, including an anti-inflammatory compound osthol, ATG5 overexpression, and C6ORF223 knockout. Overall, we revealed specific molecular mechanisms associated with RIR at the gene expression level and developed tools for discovering interventions that support the rejuvenation effect of reprogramming without posing the risk of neoplasia.
Aging is associated with the buildup of molecular damage and a gradual loss of function, culminating in chronic age-related diseases and ultimately death (1). Searching for safe and efficient interventions that can slow down or partially reverse the aging process is a major challenge in the aging field (2 – 6). In this regard, reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) has been proposed as a candidate longevity intervention due to its potential to rejuvenate cells in a targeted way (7, 8).
Pluripotency can be achieved in vitro by the ectopic expression of four transcription factors: OCT4, SOX2, KLF4, and MYC, known as OSKM or Yamanaka factors (YFs). It was demonstrated that OSKM support the generation of murine iPSCs using retroviral transduction as a delivery system and mouse embryonic fibroblasts (MEF) as the initial cell culture. Although this original experiment was inefficient in terms of the percentage of cells that terminally achieved the pluripotent state (0.1%), more advanced in vitro approaches resulted in a greatly improved efficiency, e.g. by down-regulation of methyl CpG-binding domain 3 (MBD3) levels (10). In parallel, other approaches have been developed to induce pluripotency. In particular, the expression of seven other transcription factors (7F: Jdp2-Jhdm1b-Mkk6-Glis1-Nanog-Essrb-Sall4) resulted in high efficiency of reprogramming (11).