Teaching chatbots to say “I don’t know” could curb hallucinations. It could also break AI’s business model



Today, a magnificent large-scale image of the interstellar object 3I/ATLAS, was reported here by Frank Niebling and Michael Buchner. The stacked image combines a series of 5 exposures, each lasting 3 minutes, from two telescopes (TEC 140/f5 and ASI 6200MM) between 5:08–5:22 UT on November 9, 2025.
The image shows two anti-tail jets out to 10 arcminutes towards the Sun accompanied by a longer collimated jet, extending away from the Sun out to an angular separation of 30 arcminutes, roughly the diameter of the Sun or the Moon.
At the current distance of 3I/ATLAS from Earth, 326 million kilometers, these angular extents correspond to spatial sizes of 0.95 million kilometers for the sunward anti-tail jets and 2.85 million kilometers for the tail jet away from the Sun. This enormous spatial scale is three orders of magnitude larger than the scale of the glowing halo around 3I/ATLAS in the Hubble Space Telescope image from July 21, 2025 (reported here).

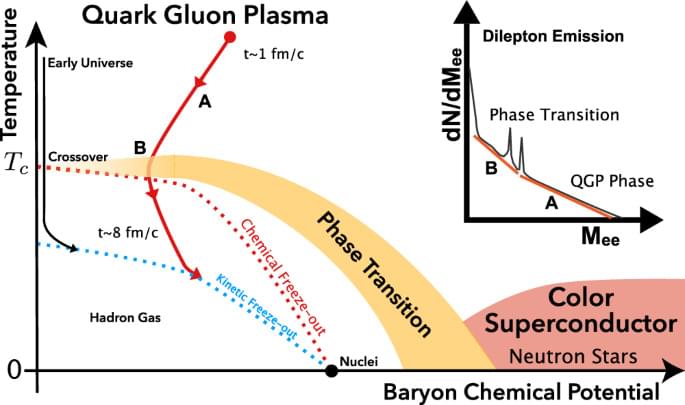


Significant work has been done to develop quantum satellites, which generate entangled pairs in space and distribute them to ground stations separated some distance away. The reverse “ uplink’’ case, where pairs are generated on the ground and swapped on the satellite using an optical Bell measurement, has not been seriously considered due to a prevailing assumption that it is practically infeasible. In this paper, we illustrate the feasibility of performing Discrete Variable photonic Bell measurements in space by conducting a detailed numerical analysis to estimate the channel efficiency and attainable pair fidelity for various satellite-station configurations. Our model accounts for a wide range of physical effects such as atmospheric effects, stray photons, and mode mismatch.

The most recent incarnation of Microsoft’s long-running flight simulator series is a genuine marvel, whether you fancy yourself an ace pilot or just want to crash spectacularly into the Eiffel Tower. Speaking more to the former instinct, Microsoft is teaming up with Boeing to put that high-fidelity simulation to work in a virtual training program for novice pilots.
As noted in a press release from Boeing, the Virtual Airplane Procedures Trainer was announced last Thursday at the European Aviation Training Summit in Portugal. The release notes the new program is “powered by Microsoft Azure and Microsoft Flight Simulator,” and is “designed to empower pilots and flight training teams with immersive, accessible and customizable tools that elevate pilot learning and readiness.”


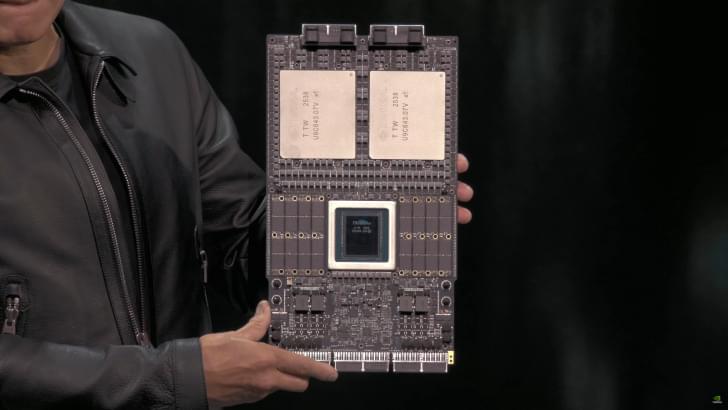
NVIDIA’s next-generation Rubin GPUs have entered production, and the company has also secured samples of HBM4 memory from all major suppliers.
A few weeks ago, NVIDIA’s CEO, Jensen Huang, showcased the next-gen Vera Rubin Superchip for the first time at GTC 2025 in Washington. We got to see two super massive GPUs stacked together with the next-generation Vera CPU, and loads of LPDDR memory on the outskirts. The Vera Rubin Superchip will lay the framework for the next wave of AI computing in data centers, and it looks like there are some good reports regarding the production timeline.