As neural interfaces, cognitive augmentation, and generative models evolve at breakneck speed, we are beginning to integrate with our own creations.




Lose an adult tooth, and you’re left with limited options that typically involve titanium implants or plastic dentures. But scientists are working on an alternative: lab-grown human teeth that could one day replace damaged ones.
Pamela Yelick and Weibo Zhang at Tufts University School of Dental Medicine in Boston have grown a mixture of pig and human tooth cells in pieces of pig teeth to create bioengineered structures that resemble real human teeth.
The toothlike structures represent a step toward bioengineered replacements for dental implants, say researchers behind the work.
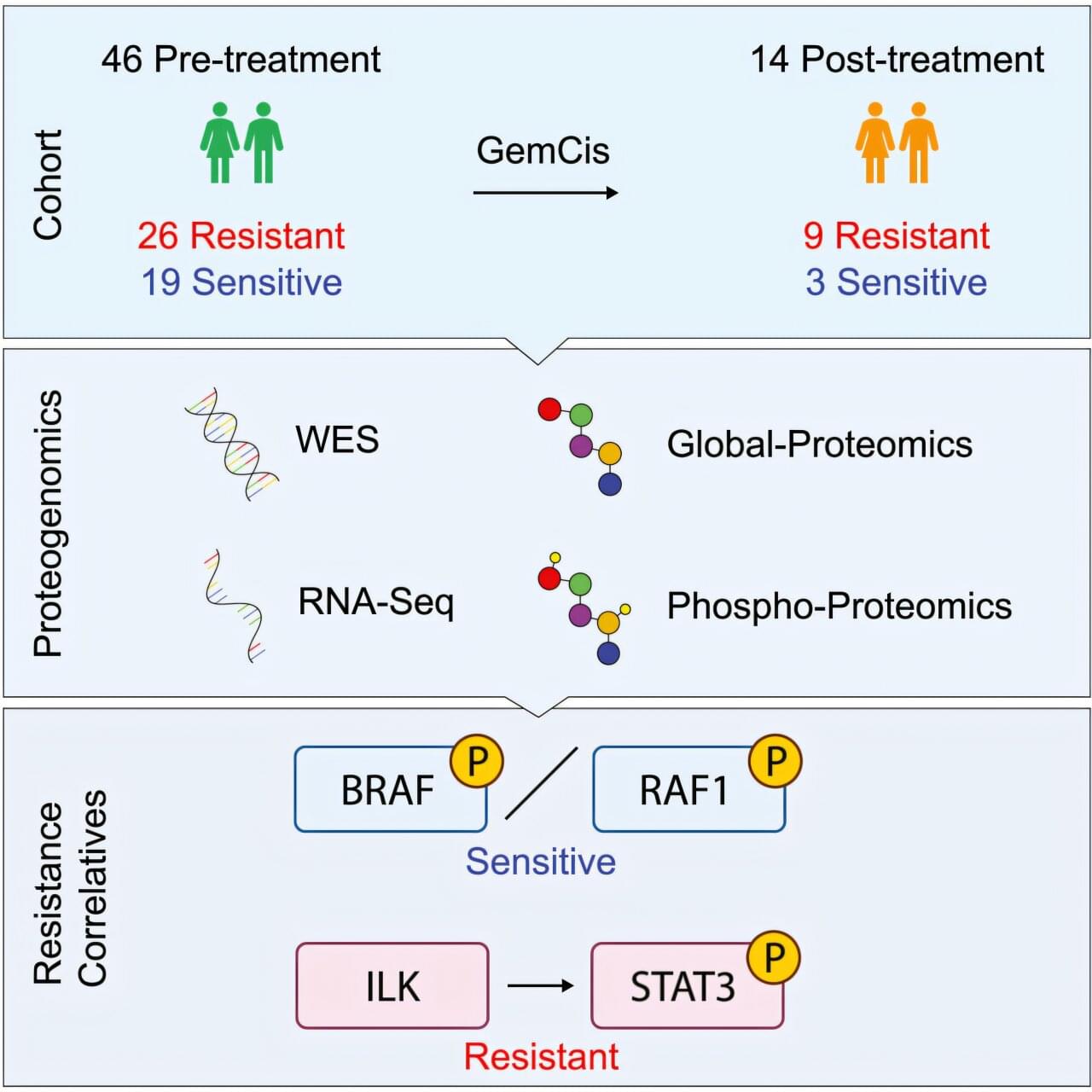
About one quarter of patients with muscle-invasive bladder cancer (MIBC) may be treated and derive a benefit with the current standard chemotherapy. To better understand why some tumors resist chemotherapy and identify better ways to treat those cancers, researchers at Baylor College of Medicine have conducted a detailed molecular analysis of MIBC tumors. The results, published in Cell Reports Medicine, offer potential new ways to identify which patients will benefit from chemotherapy and reveal possible new treatment strategies.
“One of our goals was to identify molecular markers in patient tumors that would help us predict which patients were most likely to benefit from chemotherapy and which ones might not,” said first co-author, Dr. Matthew V. Holt, director of the Lester and Sue Smith Breast Center Proteomics Laboratory at Baylor.
The researchers studied 60 MIBC tumor samples using a comprehensive multi-omics approach which included genomics (sequencing the genes of the tumor), transcriptomics (analyzing which genes are turned on or off), proteomics (the proteins produced by the tumor) and phosphoproteins (proteins with chemical tags that control their activity).

Please find under this blog the latest updates on exciting news happening every day in the world of Materials Science and Materials Chemistry research and development (with a special emphasis on the Computational aspects of these research fields), via our diverse selection of news articles! Many thanks for your interest and support, Dr. Gabriele Mogni Email contact: [email protected] Website: www.qscomputing.com
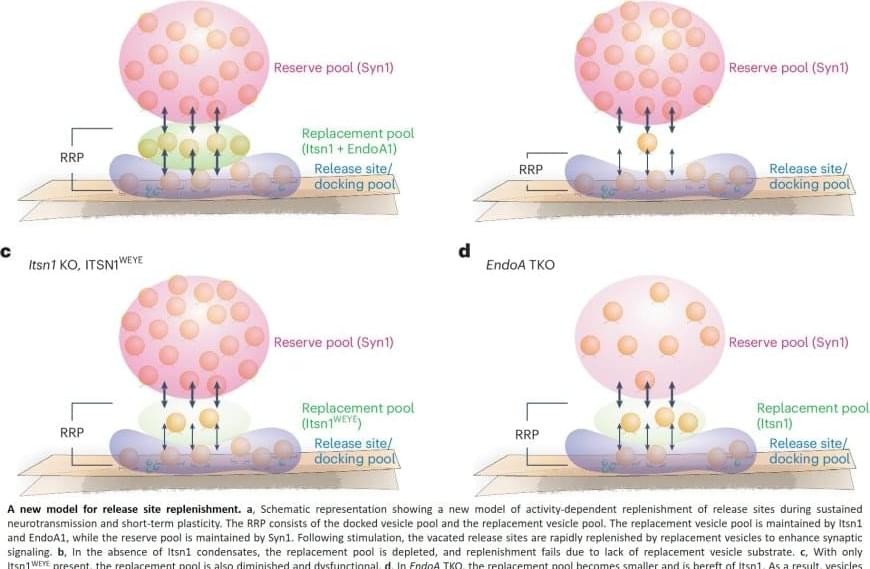
Message transfer from brain cell to brain cell is key to information processing, learning and forming memories. The bubbles, synaptic vesicles, are housed within the synapse — the connection point where brain cells communicate. In typical synapses within the brains of mammals, 300 synaptic vesicles are clustered together in the intersection between any two brain cells, but only a few of these vesicles are used for such message transfer, researchers say. Pinpointing how a synapse knows which vesicles to use has long been a target of research by those who study the biology and chemistry of thought.
In an effort to better understand the operation of these synaptic vesicles, the team designed a study that first focused on endocytosis, a process in which brain cells recycle synaptic vesicles after they are used for neuronal communication.
Already aware of intersectin’s general role in endocytosis and neuronal communication, the scientists genetically engineered mice to lack the gene that codes for intersectin. However, and somewhat to their surprise, the lead says removing the protein did not appear to halt endocytosis in brain cells.
The research team refocused their experiments, taking a closer look at the synaptic vesicles themselves.
Using a high-resolution fluorescence microscope to observe where intersectin is in a synapse, the researchers found it in between vesicles that are used for neuronal communication and those that are not, as if they are physically separating the two.
To further understand the role of intersectin at this location, they used an electron microscope to visualize synaptic vesicles in action across one billionth of a meter. In all the nerve cells from mice lacking this protein, the scientists say synaptic vesicles close to the membrane were absent from the release zone of the synapse, the place where the bubbles would discharge to nearby neurons.
“This suggested that intersectin regulates release, rather than recycling, of these vesicles at this location of the synapse,” says the author.
So I took @meta CEO Mark Zuckerberg blog post on’superintelligence’ aka #artificialgeneralintelligence from https://www.meta.com/superintelligence/ and generated an audio track using @Descript (no edits were made) and then created a ‘data’-like avatar (which is often compared to what Zuck used to look like::). Then I asked Descript to add some stock footage and generate captions… (again, no edits) and here we are.
You can judge it for yourself, but here’s what I think:
1) Zuck’s memo is… crap (sorry)
2) The voiceover is decent but yes… crappily generic… and of course, it didn’t allow me to use Z’s voice or image.
3) The stock footage used (I did not make ANY edits) is… crap (to call it generic would be generous)
4) The cuts, layovers and transitions are…crap.
The result is crap. I hope you don’t like it.
Note: This is NOT by any means a comment on the power of @Descript’s AI editing platform which I really like and constantly use, and definitely recommend. But it’s not a miracle machine:)
To see what Descript can do if you put real effort into it, go here: https://youtu.be/AEKtY7F5Z0Q?si=pIYoqoms7PfMukoo.
Rather, it shows that mindlessness, lazyness, and effortlessness creates SLOP. As creators, we must resist using these tools without serious editing, questioning and curating. #thehumanresistance.
The summary below was also written by AI:

Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
Scientists have found a way to supercharge lung cancer treatment by transplanting healthy mitochondria into tumors, which both boosts immune response and makes chemotherapy far more effective. By combining this novel method with cisplatin, researchers reversed harmful tumor metabolism and empowered immune cells to fight back, all without added toxicity.