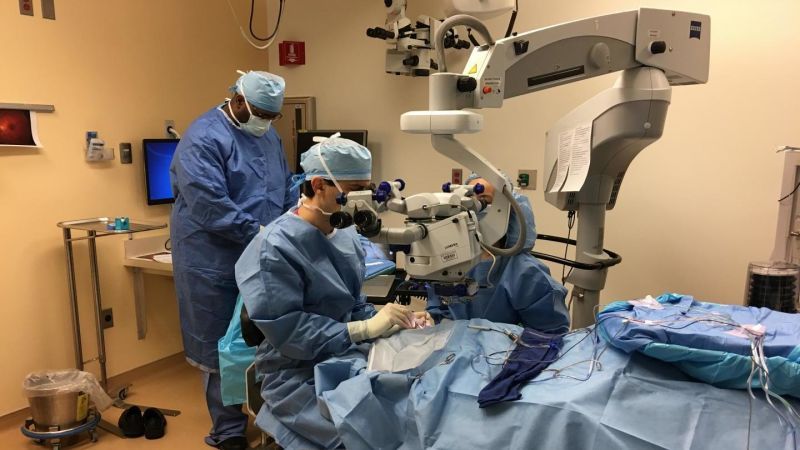
On Tuesday, a 13-year-old boy from New Jersey was at the center of medical history as he became the first person in the US to receive an FDA-approved gene therapy for an inherited disease. The event marks the beginning of a new era of medicine, one in which devastating genetic conditions that we are born with can be simply edited out of our DNA with the help of modern biomedical technologies.
The therapy, Luxturna, from Spark Therepeutics, was approved by the FDA in December to treat a rare, inherited form of blindness. Its price tag, set at $850,000—or $425,000 per eye—made it the most expensive drug in the US and sparked mass sticker-shock. But the therapy, which in high-profile clinical trials has allowed patients to see the stars for the first times, also offered the almost miraculous possibility of giving sight to the blind.
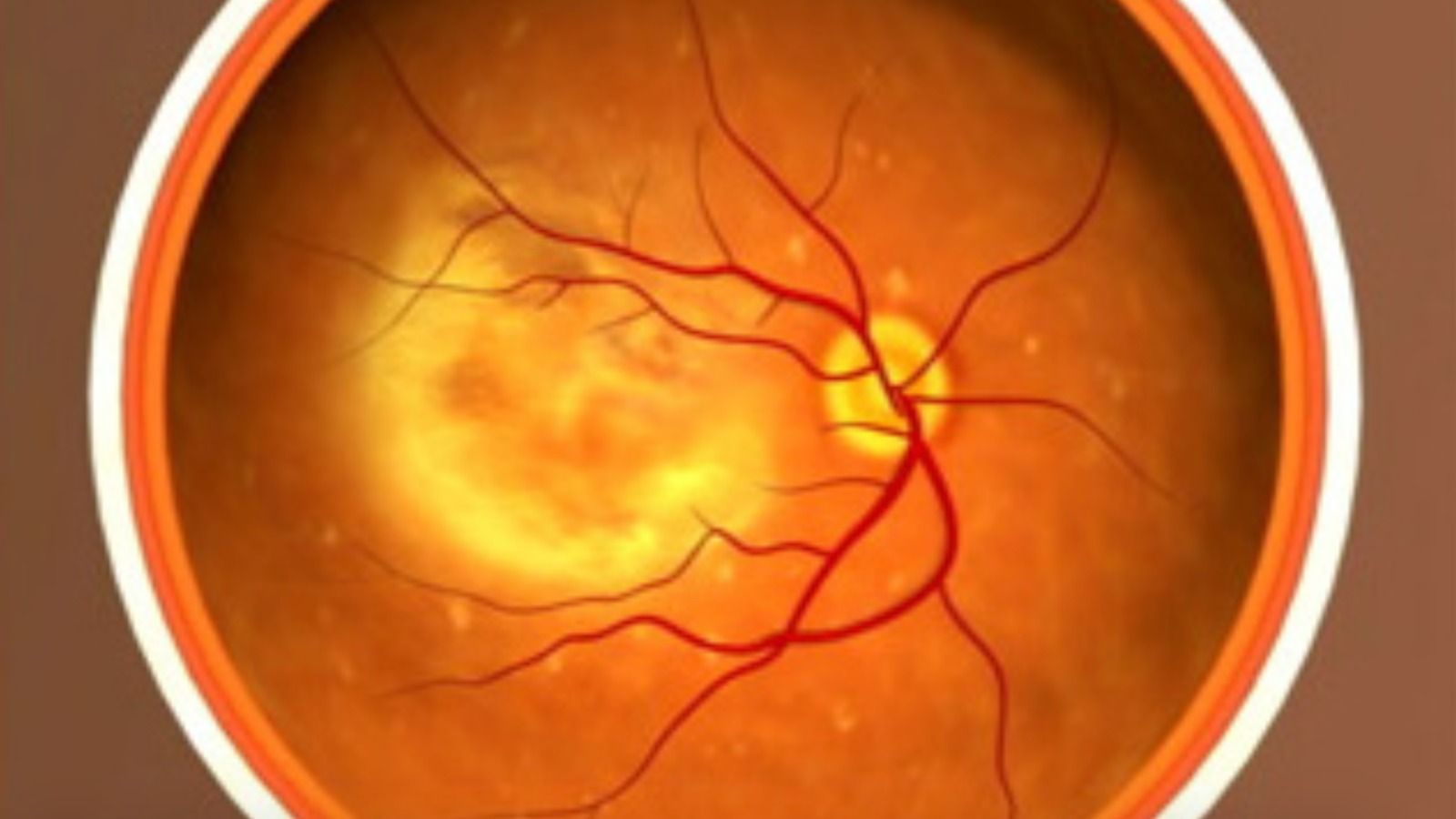
The therapy is intended to treat retinal diseases, including leber congenital amaurosis or retinitis pigmentosa, caused by mutations in the RPE65 gene. The RPE65 gene produces an enzyme that helps the eye process light. In these disorders, severe visual impairment begins often in infancy, and sometimes degrades over time. Some people with a mutated copy of the gene can see during the day; others are legally blind. The drug works by delivering a correct copy of the RP65 gene to retinal cells, allowing the patient to produce the deficient enzyme—and, hopefully, restoring their vision. (Luxturna is considered by some to be the first “true gene therapy” approved by the FDA, since other approved therapies, like those for blood cancers, involve removing a patient’s cells from their body, modifying them externally, and then infusing them back into the body.)
Continue reading “13-Year-Old Boy Is First Person in US to Receive Newly Approved Gene Therapy for Blindness” »