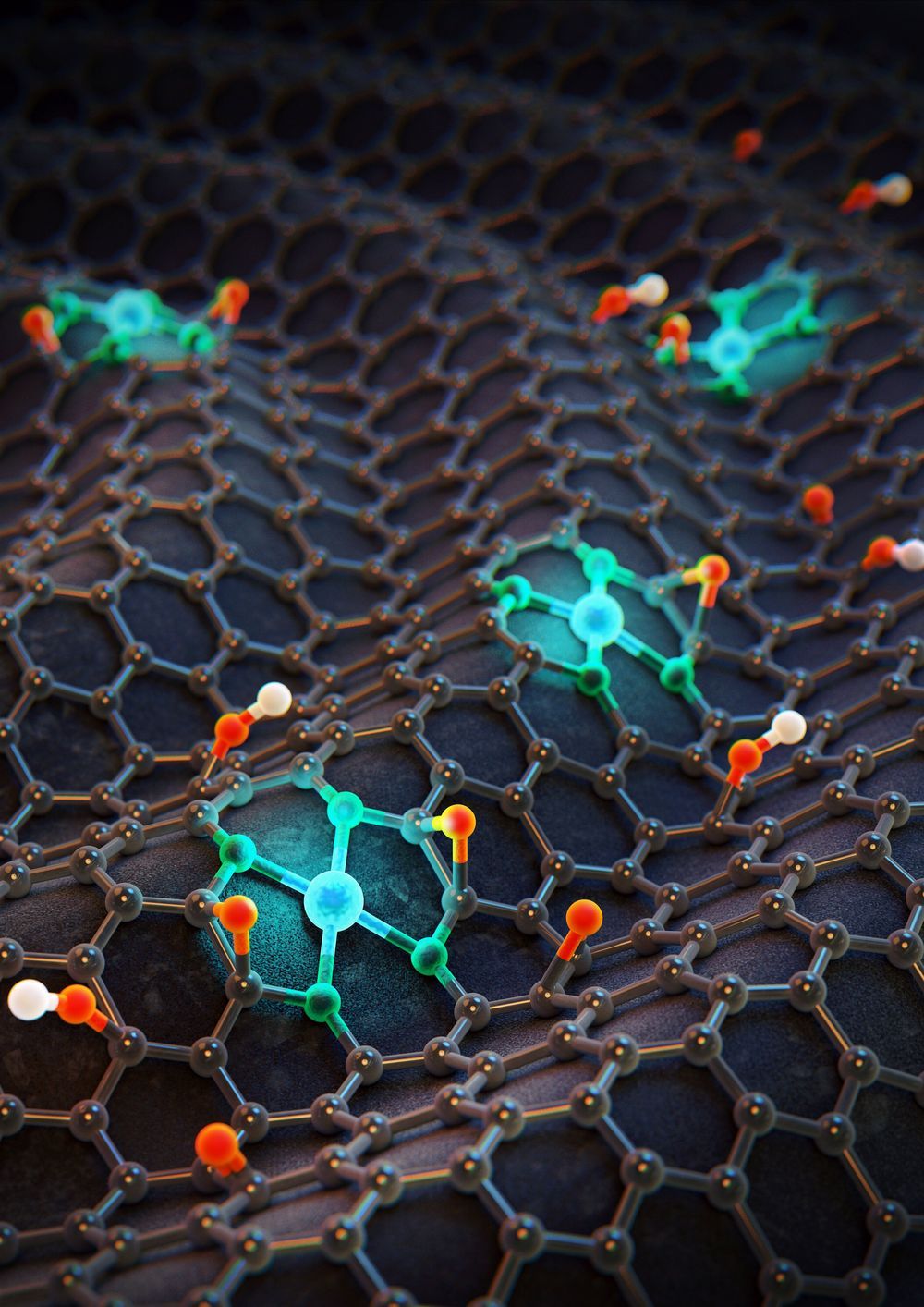
IBS scientists and their colleagues have recently report an ultimate electrocatalyst that addresses all of the issues that trouble H2O2 production. This new catalyst comprising the optimal Co-N4 molecules incorporated in nitrogen-doped graphene, Co1-NG(O), exhibits a record-high electrocatalytic reactivity, producing up to 8 times higher than the amount of H2O2 that can be generated from rather expensive noble metal-based electrocatalysts.
Just as we take a shower to wash away dirt and other particles, semiconductors also require a cleaning process. However, its cleaning goes to extremes to ensure even trace contaminants “leave no trace.” After all the chip fabrication materials are applied to a silicon wafer, a strict cleaning process is taken to remove residual particles. If this high-purity cleaning and particle-removal step goes wrong, electrical connections in the chip are likely to suffer from it. With ever-miniaturized gadgets on the market, the purity standards of the electronics industry reach a level equivalent to finding a needle in a desert.
That explains why hydrogen peroxide (H2O2), a major electronic cleaning chemical, is one of the most valuable chemical feedstocks that underpins the chip-making industry. Despite the ever-growing importance of H2O2, its industry has been left with an energy-intensive and multi-step method known as the anthraquinone process. This is an environmentally unfriendly process which involves the hydrogenation step using expensive palladium catalysts. Alternatively, H2O2 can be synthesized directly from H2 and O2 gas, although the reactivity is still very poor and it requires high pressure. Another eco-friendly method is to electrochemically reduce oxygen to H2O2 a via 2-electron pathway. Recently, noble metal-based electrocatalysts (for example, Au-Pd, Pt-Hg, and Pd-Hg) have been demonstrated to show H2O2 productivity although such expensive investments have seen low returns that fail to meet the scalable industry needs.