Self-assembly is ubiquitous in the natural world, serving as a route to form organized structures in every living organism. This phenomenon can be seen, for instance, when two strands of DNA—without any external prodding or guidance—join to form a double helix, or when large numbers of molecules combine to create membranes or other vital cellular structures. Everything goes to its rightful place without an unseen builder having to put all the pieces together, one at a time.
For the past couple of decades, scientists and engineers have been following nature’s lead, designing molecules that assemble themselves in water, with the goal of making nanostructures, primarily for biomedical applications such as drug delivery or tissue engineering. “These small-molecule-based materials tend to degrade rather quickly,” explains Julia Ortony, assistant professor in MIT’s Department of Materials Science and Engineering (DMSE), “and they’re chemically unstable, too. The whole structure falls apart when you remove the water, particularly when any kind of external force is applied.”
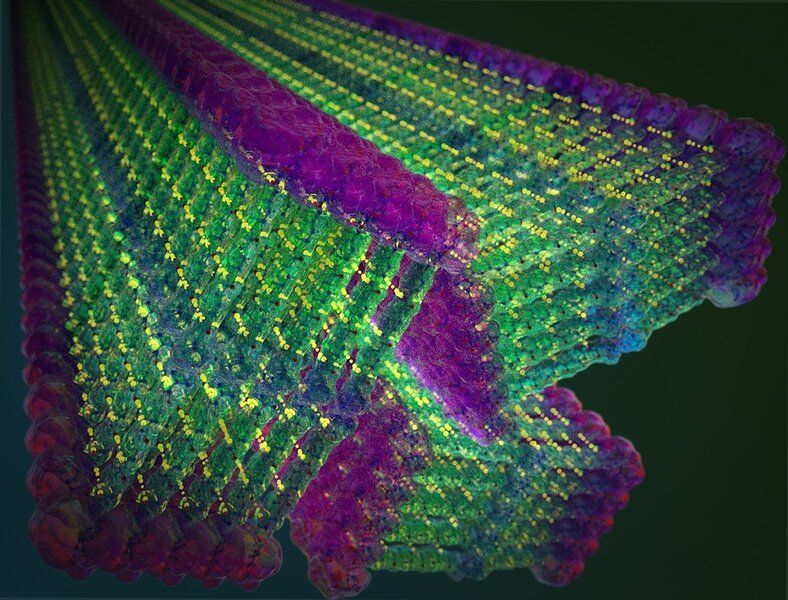
She and her team, however, have designed a new class of small molecules that spontaneously assemble into nanoribbons with unprecedented strength, retaining their structure outside of water. The results of this multi-year effort, which could inspire a broad range of applications, were described on Jan. 21 in Nature Nanotechnology by Ortony and coauthors.